Why do cells, and by extension humans, age? The answer may have a lot to do with mitochondria, the organelles that supply energy to cells. Although that idea is not new, direct evidence in human cells was lacking. Until now.
In a study published in Communications Biology , a team led by researchers at Columbia University found that human cells with damaged mitochondria respond by speeding up and expending more energy. While this adaptation, called hypermetabolism, improves the short-term survival of cells, it comes at a high cost: a dramatic increase in the rate at which cells age.
"The findings were made in cells from patients with rare mitochondrial diseases , but may also have relevance to other conditions that affect mitochondria, including neurodegenerative diseases, inflammatory conditions, and infections," says lead investigator Martin Picard, PhD, associate professor of behavior. medicine (in psychiatry and neurology) at Columbia University Vagelos College of Physicians and Surgeons.
“In addition, hypermetabolism may be a key reason why most cells deteriorate as we age.”
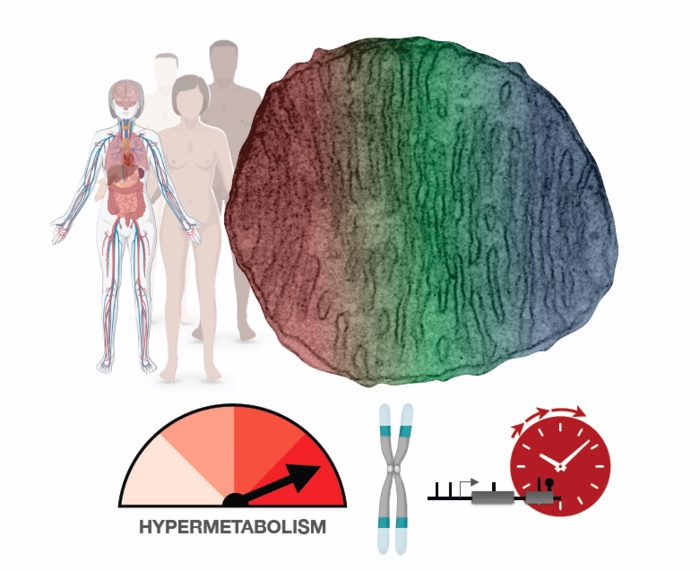
Mitochondrial defects caused by rare genetic mutations cause human cells to increase their metabolism. While that helps with short-term survival, it comes at a high cost: a dramatic increase in the rate at which cells age. Hypermetabolism may also be a key reason why most cells deteriorate as we all age. CREDIT: Columbia University Irving Medical Center (Martin Picard)
Hypermetabolic cells age faster
It was generally assumed that mitochondrial defects (which impair the conversion of food sources into usable energy) would force cells to slow their metabolic rate in an effort to conserve energy. However, when analyzing metabolic activity and energy consumption in cells from patients with mitochondrial diseases, the researchers found that cells with impaired mitochondria double their energy expenditure. Furthermore, reanalyzing data from hundreds of patients with different mitochondrial diseases showed that mitochondrial defects also increase the energetic cost of life at the whole-body level.
Although this increase in energy keeps cells functioning, it also degrades cells’ telomeres (caps that protect the ends of our chromosomes) and activates responses to stress and inflammation. The net effect accelerates biological aging.
"When cells expend more energy to produce proteins and other substances essential for short-term survival, they are likely to steal resources from processes that ensure long-term survival, such as telomere maintenance," says Gabriel Sturm, student graduate and lead author of this study.
Hypermetabolism, fatigue and aging
This hypermetabolic state could explain why people with mitochondrial diseases experience fatigue and exercise intolerance , among other symptoms. “To compensate for the extra energy use in your cells, your body ’tells’ you not to push yourself too hard, to conserve energy. “We are likely to see the same dynamic as people age and their vitality declines ,” says Picard.
The study does not point to any new remedies for patients with mitochondrial diseases, which are currently not treatable, but reinforces current recommendations for patients to move more. “That may seem counterintuitive, since if you are more active, you will expend more energy and possibly make your symptoms worse,” Sturm says. “But exercise is known to increase the efficiency of an organism. “A person who runs, for example, uses less energy to sustain basic bodily processes than someone who is not physically active.”
Improving the body’s efficiency, which would reduce energy use in cells and improve fatigue and other symptoms, may partially explain the health benefits of exercise in patients with mitochondrial diseases and healthy people.
In their search for new treatments for mitochondrial diseases, researchers should focus on hypermetabolism, Picard says. “Although mitochondrial defects affect the ability of cells to produce energy, energy deficiency may not be the primary initiator of the disease. Our study shows that these defects increase energy consumption. To move the needle therapeutically, we may need to target hypermetabolism. “We need more research to know if that would work.”
Hypermetabolism is also common to other diseases. If increased cellular energy expenditure plays a causal role in driving the aging process, targeting hypermetabolism may be a way to improve fatigue, improve people’s quality of life, or even delay biological aging.















