Summary A spinal cord injury disrupts communication between the brain and the region of the spinal cord that produces walking, leading to paralysis. Here, we restored this communication with a digital bridge between the brain and spinal cord that enabled a person with chronic tetraplegia to stand and walk naturally in community settings. This brain-spine interface (BSI) consists of fully implanted stimulation and recording systems that establish a direct link between cortical signals and analog modulation of epidural electrical stimulation directed to the regions of the spinal cord involved in the production of spinal cord. March. A highly reliable brain-spine interface (BSI) calibrates in just a few minutes. This reliability has remained stable for a year , even during independent use at home. The participant reports that the brain-spine interface (BSI) allows him natural control over his leg movements to stand, walk, climb stairs, and even traverse complex terrain. Furthermore, BSI-supported neurorehabilitation improved neurological recovery. The participant regained the ability to walk with crutches on the ground even when the BSI was turned off. This digital bridge establishes a framework for restoring natural control of movement after paralysis. |
The treatment
To walk, the brain sends executive commands to neurons located in the lumbosacral spinal cord. Although most spinal cord injuries do not directly damage these neurons, disruption of the descending pathways disrupts the brain-derived commands that are necessary for these neurons to produce walking. The consequence is permanent paralysis .
We previously demonstrated that epidural electrical stimulation directed to individual dorsal root input zones of the lumbosacral spinal cord allows modulation of specific motor groups of the legs. In turn, recruitment of these dorsal root input zones with preprogrammed spatiotemporal sequences replicates the physiological activation of leg motor groups underlying standing and walking. These stimulation sequences restored standing and basic walking in people with paralysis due to spinal cord injury. However, this recovery required wearable motion sensors to detect motor intentions from residual movements or compensatory strategies to initiate preprogrammed stimulation sequences. Consequently, gait control was not perceived as completely natural . Furthermore, participants showed a limited ability to adapt leg movements to changing terrain and volitional demands.
Here, we suggest that a digital bridge between the brain and spinal cord would allow voluntary control over the timing and amplitude of muscle activity, restoring more natural and adaptive control of standing and walking in people. with paralysis due to spinal cord injury.
Digital bridge from the brain to the spinal cord
To establish this digital bridge, we integrated two fully implanted systems that allow recording of cortical activity and stimulation of the lumbosacral spinal cord wirelessly and in real time (Fig. 1a).
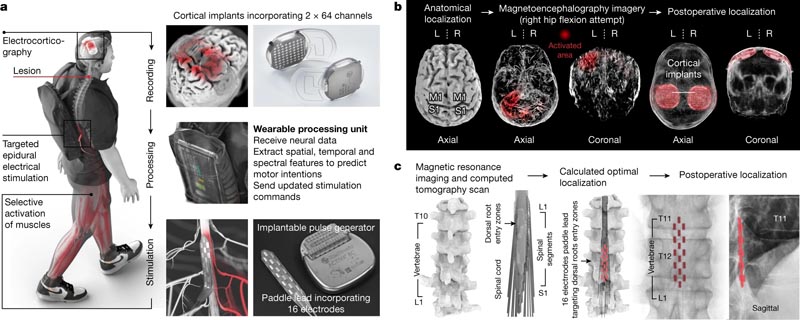
Fig. 1: Design, technology and implementation of the BSI. a , Two cortical implants composed of 64 electrodes are placed epidurally over the sensorimotor cortex to collect ECoG signals. A processing unit predicts motor intentions and translates these predictions into the modulation of epidural electrical stimulation programs directed to the dorsal root input zones of the lumbosacral spinal cord. Stimuli are delivered using an implantable pulse generator connected to a 16-electrode paddle lead. b , Images informing preoperative planning of cortical implant locations and postoperative confirmation. L, left; A, correct. c , Custom computational model predicting optimal paddle wire placement to target dorsal root entry zones associated with lower extremity muscles and postoperative confirmation.
This integrated chain of hardware and software established a wireless digital bridge between the brain and spinal cord: a brain-spine interface (BSI) that converts cortical activity into analog modulation of epidural electrical stimulation programs to tune the activation of the muscles of the lower extremities and thus regain standing and walking after paralysis due to spinal cord injury.
Discussion
We conceived a wireless digital bridge between the brain and spinal cord that restored natural control over lower extremity movements for standing and walking on complex terrain after paralysis due to spinal cord injury. Additionally, neurorehabilitation mediated neurological improvements that persisted even when the bridge was turned off.
Validation of this digital bridge was restricted to a single individual with severe but partial spinal cord damage and therefore it is unclear whether the brain-spine interface (BSI) will be applicable to other injury locations and severities. However, several observations suggest that this approach will be applicable to a broad population of people with paralysis. First, the physiological principles underlying targeted epidural electrical stimulation of the spinal cord have now been validated in nine out of nine people treated with incomplete and complete lesions. Second, we developed procedures that supported simple, rapid, and stable calibration of the link between cortical activity and stimulation schedules, allowing the participant to operate the BSI at home without supervision. Third, comparable robustness and stability of this computational and technological brain decoding framework has now been observed in two additional people with tetraplegia. Although the participant’s prior experience with stimulation accelerated the setup of the BSI, we do not foresee major impediments to implementing a BSI in new individuals. In fact, we were able to set up stimulation programs that restored walking within one day in three participants with complete sensorimotor paralysis.
The concept of a digital bridge between the brain and spinal cord heralds a new era in the treatment of motor deficits due to neurological disorders.
















