Wound healing is achieved through the interaction of three key components : a set of precursor cells that can proliferate and differentiate into fibroblasts and keratinocytes; neoangiogenesis to restore blood flow to the injury and provide nutrients and cells to the wound; and a competent immune system capable of generating a controlled inflammatory response. When these components fail, wound healing stops and a chronic non-healing wound results. Chronic wounds are characterized by stopping the progression of the wound healing phases, specifically in the inflammatory phase.
Infections are a common trigger for the development of chronic wounds and complicate the healing of those wounds that are already arrested in the inflammatory phase. Because skin and wounds are non-sterile environments , it is widely accepted that wound infections occur on a spectrum ranging from contamination , colonization , local infection , and spread of infection to systemic infection. Therefore, a common challenge for clinicians is to differentiate between contaminated and colonized wounds and wounds with subtle local infections to provide timely treatment before the infection becomes a more significant problem. Unfortunately, because clinical inspection alone has demonstrated less than 60% accuracy in identifying infected wounds, there is a pressing need to identify diagnostic adjuncts that can help achieve better outcomes.
Traditionally, a microbiological evaluation of the wound and perilesional areas is used to rule out the presence of infections. However, cultures, molecular techniques, and other conventional diagnostic results take time and are sometimes inaccessible and expensive. Infrared thermography ( IRT) has shown promise as a tool to help diagnose inflammation and infection in wounds and skin disorders, as IRT heat signals have shown a high degree of correlation with inflammatory changes in the skin and processes deep infections.
However, while thermal changes indicate inflammation as an indicator of infection, these changes cannot be used to diagnose the presence of an infectious process. Another point-of-care technology that has shown great potential for identifying subtle infectious processes is the use of violet light to elicit bacterial fluorescence (BF) in wounds. When wound bioburdens <10 4 -10 5 bacteria are present in the wounds, BF can be used to identify their presence as a red signal for porphyrin-producing organisms or a cyan signal for those bacteria that produce pyoverdine pigments, with a precision of approximately 70%. However, it should be noted that BF can only identify bacteria present on the surface of wounds, as this imaging technology only penetrates <1.5 mm into the tissues, therefore any deeper bacterial contamination or infections due to to other agents, such as fungi. Therefore, despite the promising results these technologies have demonstrated in assessing the presence of wound infections, their use alone has significant shortcomings and the combined use of IRT and BF has not been explored.
The Swift Ray 1 (Swift Medical, Toronto, ON) is a novel point-of- care hyperspectral imaging (HSI) device that enables acquisition of medical-grade images via smartphone. HSI acquires a multidimensional image data set (one dimension per imaging modality), called a hypercube , that provides diagnostic information about tissue physiology, morphology, and composition. The Ray 1 device is equipped with near- and long-wave infrared sensors, violet light sources, and visible-range LEDs, allowing simultaneous acquisition of visible light, IRT, and BF images as a hypercube. It also integrates into the Swift Skin and Wound app (Swift Medical, Toronto, ON), allowing for accurate wound area measurement, temperature quantification, and fluorescence area quantification. Under the hypothesis that, through the analysis of HSI data acquired with the Ray 1 device, wounds can be classified as without associated inflammatory response, with inflammatory response or infected , the objective of this study was to analyze a series of HSI images of patients to determine if there are differences in the images between infected and non-infected wounds.
Background
Clinical signs and symptoms (CSS) of infection are a standard part of wound care, however, they may have low specificity and sensitivity , which may vary further due to physician knowledge, experience, and education. Wound photography is increasingly being adopted to support wound care. Thermography has been studied in the medical literature to evaluate signs of perfusion and inflammation for decades. Bacterial fluorescence has recently emerged as a valuable tool for detecting high bacterial load in wounds. The combination of these modalities offers a potential objective detection tool for wound infection.
Methods:
A multicenter prospective study of 66 outpatients receiving wound care used hyperspectral imaging to collect visible light, thermography, and bacterial fluorescence images. Wounds were assessed and examined using the International Wound Infection Institute (IWII) checklist for CSS of infection. Principal component analysis was performed on the images to identify wounds that presented as infected, inflamed, or noninfected.
Results:
The model was able to accurately predict all three classes of wounds ( infected, inflamed and non-infected ) with an accuracy of 74%. They performed better in infected wounds (100% sensitivity and 91% specificity) compared to noninflamed (94% sensitivity, 70% specificity) and inflamed wounds (85% sensitivity, 77% specificity).
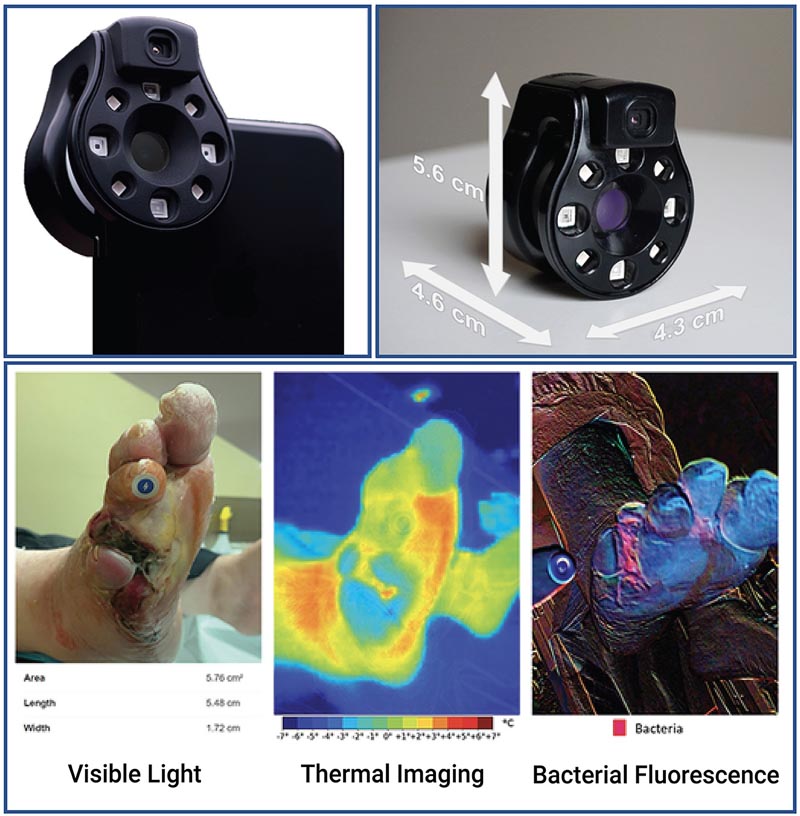
Figure The Ray 1 Hyperspectral Imaging Device. The Ray 1 Imaging Device is a pocket-sized hyperspectral camera designed to fit over a smartphone camera lens and connect wirelessly to Swift Medical’s Skin and Wound app. Once connected, the camera allows for the simultaneous acquisition of: visible light images that can be used for clinical inspection, wound area measurement, and automated identification of tissue types present in the wound; infrared thermal images for the evaluation of vascular and inflammatory patterns; and bacterial fluorescence images for the evaluation of bacterial biological load in wounds, as in the vignette presented at the bottom of the figure .
Discussion:
The combination of multiple imaging modalities allows the application of models to improve wound assessment. Detection of infections using clinical signs and symptoms (CSS) is vulnerable to subjective interpretation and variability depending on the education and skills of clinicians.
Allowing clinicians to use hyperspectral imaging at the point of care may allow for earlier detection and intervention of infections, possibly preventing delays in wound healing and minimizing adverse events.
Comments
It is very difficult for doctors to identify a wound that is becoming infected. Clinical signs and symptoms are imprecise and methods to identify bacteria can be time-consuming and inaccessible, so diagnosis can be subjective and depend on the doctor’s experience. But the infection can stop healing or spread to the body if not treated quickly, seriously endangering the patient’s health. An international team of scientists and doctors believe they have the solution: a device that runs from a smartphone or tablet app, allowing advanced imaging of a wound to identify an infection.
"Wound care is one of the most costly and overlooked threats to patients and our healthcare system as a whole," said Robert Fraser of Western University and Swift Medical Inc., corresponding author of the study published in Frontiers in Medicine . "Doctors need better tools and data to better care for their patients who are suffering unnecessarily."
Shedding light on injuries
Scientists developed a device called the Swift Ray that can connect to a smartphone and the Swift Skin and Wound software . This can take medical-grade photographs, infrared thermography images (which measure body heat), and bacterial fluorescence images (which reveal bacteria using violet light).
None of these images would be sufficient to identify an infection on their own. Clinical inspection has low precision, as does thermography which measures heat changes caused by inflammation and infection. Bacterial fluorescence can only observe the surface of a wound, which is naturally contaminated with bacteria, so additional methods are needed to differentiate between contamination and an infected wound.
"Research has shown that bacterial imaging helps guide doctors’ work to remove non-viable tissue, but cannot identify infection on its own," explained Dr. José Ramírez-García Luna of the Health Center of the McGill University, first author of the study. "Thermography provides information about the inflammatory and circulatory changes that occur under the skin."
Scientists sought to combine these modalities to create a method that did not require multiple expensive devices, overcame the weaknesses of each imaging method, and could provide an objective measure of wound healing.
To test their device, they recruited 66 injured patients. Their wounds showed no signs of the infection spreading further, contained no foreign bodies, and had not been previously treated with antibiotics or growth factors. Patients’ wounds were uncovered, cleaned and dried before imaging, and then cared for as usual.
An image of health
The images were reviewed by an investigator who was not present in the wound care process. Four patterns were identified.
Wounds that were no warmer than healthy skin and had no bacterial fluorescence were considered "noninflamed ," while wounds that were slightly warmer than healthy skin and had no or slight bacterial fluorescence were considered "inflamed ." " . The latter two patterns (substantially warmer wounds, with or without bacterial fluorescence) were designated as "infected ," because all doctors who had examined these wounds had considered them infected.
Of the 66 wounds, 20 were considered noninflamed, 26 inflamed, and 20 infected.
The researchers performed a principal component analysis and used an algorithm to see if a machine learning model could accurately identify these different categories of wounds. They found that the model could identify all three very well, with an overall accuracy of 74%. When differentiating between infected and non-infected wounds , the model correctly identified 100% of infected wounds and 91% of non-infected wounds.
A new tool in the briefcase
The researchers noted that images should always be considered in their medical context . For example, a wound that is cold enough to be classified as non-inflamed could have a limited blood supply, compromising healing.
But because Swift Ray combined with Swift Skin and Wound software allows clinicians to combine multiple infection identification modalities, it increases the tools available without requiring the use of multiple expensive devices. In the future, it could make it possible to ensure rapid and accurate diagnosis for every injured patient and enable more effective telemedicine assessments.
"This was a pilot study and follow-up studies are planned," Fraser cautioned. "In the future, patient populations with more types of wounds will be required to validate across populations."
Final message In conclusion, combining wound imaging with visible light increases the sensitivity and specificity of infection detection and helps classify uninfected wounds as inflamed or noninflamed. This categorization can then be used to provide more rational and specific treatment, assess the causes of non-healing as perfusion-based or infection-based, assess the need to change the type of wound dressings, and monitor response to treatment. treatments. Therefore, the advent of this pocket-sized imaging device capable of delivering this information as an “all-in-one” device holds great promise for enabling point-of-care assessment of perfusion, inflammation, and infection. |















