Influence of lighting conditions on substrate metabolism, energy expenditure and thermoregulation in insulin-resistant individuals
Highlights
|
Light is the most important timing cue, i.e. zeitgeber , for synchronizing the internal biological clock of mammals with the 24-hour environmental light/dark cycle. The suprachiasmatic nucleus located in the hypothalamus acts as a master pacemaker by integrating light/dark information received from the retina with endogenously generated rhythms in neuronal and humoral signals, which in turn can synchronize peripheral clocks in organs such as the liver, muscle skeletal and pancreas.
In this way, the body can anticipate the different metabolic demands associated with a certain time slot within the light/dark cycle, such as feeding and fasting, physical activity and rest. Optimizing indoor light conditions to more closely mimic the natural light/dark cycle is likely to ensure proper regulation of behavioral rhythms, including sleep/wake and feeding cycles, and help maintain metabolic health.
However, in our modern society, artificial light is available 24 hours a day and most people are exposed to electric light and light-emitting displays during the dark period of the natural light/dark cycle. In humans, detrimental effects of nighttime light (LAN) include acutely elevated postprandial glucose and insulin levels and elevated postprandial insulin and glucagon-like peptide-1 levels after breakfast following overnight light exposure. .
In addition to LAN, our modern society also faces the lack of sufficient time under bright light conditions during the day and, in fact, most of the time is spent indoors under artificial lighting (under much lower light levels in compared to natural daylight outdoors). Exposure to bright light before and during breakfast increased postprandial glucose and triacylglycerol (TG) levels in patients with type 2 diabetes mellitus, while it only increased TG levels in healthy young men.
Some studies investigated experimental light exposure protocols for 24 h and suggested that bright light can influence glucose metabolism in healthy young volunteers at different times of the day compared to dim light. However, whether optimizing artificial light exposure over a 24-h period can improve whole-body energy and substrate metabolism and glucose homeostasis in people with resistance to light has not yet been investigated. insulin.
Objectives/hypotheses
In our modern society, artificial light is available 24 hours a day and most people are exposed to electric light and light-emitting displays during the dark period of the natural light/dark cycle.
Such suboptimal lighting conditions have been associated with adverse metabolic effects, and redesigning indoor lighting conditions to mimic the natural light/dark cycle promises to improve metabolic health.
Our objective was to compare metabolic responses to lighting conditions that resemble the natural light/dark cycle in contrast to suboptimal lighting in people at risk of developing metabolic diseases.
Methods
Therefore, here we performed a non-blinded, randomized, controlled, crossover trial in which overweight insulin-resistant volunteers (n = 14) were exposed to two 40-h laboratory sessions with different 24-h illumination protocols while They remained in a metabolic chamber under real conditions.
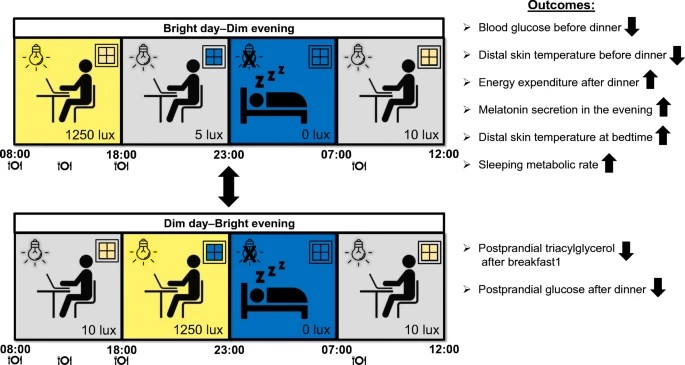
In the bright day-dim night condition , volunteers were exposed to bright electric light (~1250 lx) during the day (08:00-18:00 h) and dim light (~5 lx) during the night (18:00 h). –11:00 p.m.).
Vice versa, in the Dim Day condition. Bright night, volunteers were exposed to dim light during the day and bright light at night.
Randomization and assignment to light conditions were performed by sequential numbering. During both illumination protocols, we performed 24-h indirect calorimetry and continuous measurements of core body and skin temperature, and took frequent blood samples.
The primary outcome was plasma glucose focusing on the preprandial and postprandial periods of the intervention.
Results
Spending the day in bright light resulted in a greater increase in postprandial triacylglycerol levels after breakfast, but lower glucose levels before dinner at 6:00 p.m., compared with dim light (5.0 ± 0 .2 vs. 5.2 ± 0.2 mmol/l, n = 13, p = 0.02).
The Dim Day-Bright Night group reduced the increase in postprandial glucose after dinner compared to the opposite group (incremental AUC: 307 ± 55 vs. 394 ± 66 mmol/l × min, n = 13, p = 0.009) .
After the Bright Day-Dark Night condition, sleep metabolic rate was identical compared to the baseline night, while it decreased after Dark Day-Bright Night.
Melatonin secretion at night was strongly suppressed for dark day-bright night but not for bright day-dark night.
Distal skin temperature for bright day-dim night was lower at 18:00 h (28.8 ± 0.3 °C vs. 29.9 ± 0.4 °C, n = 13, p = 0.039 ) and higher at 11:00 p.m. compared to the evening dim-bright day (30.1 ± 0.3 °C vs. 28.8 ± 0.3 °C, n = 13, p = 0.006).
Fasting and postprandial plasma insulin levels and respiratory exchange rate were not different between the two lighting protocols at any time.
Conclusions/interpretation
Together, these findings suggest that the indoor light environment modulates postprandial substrate handling, energy expenditure, and thermoregulation of insulin-resistant volunteers as a function of time of day.
Discussion
Optimizing indoor lighting conditions to more closely mimic the natural light/dark cycle promises to prevent the adverse effects on whole-body energy and glucose metabolism associated with the constant indoor lighting conditions that prevail today.
Here, we found that spending the day in bright light led to lower plasma glucose levels before the last meal of the day, while plasma glucose levels after that meal were higher after the bright-day condition. dim night, that is, when dinner was consumed under low light conditions.
As expected, bright day-dark evening facilitated melatonin secretion at night, which was strongly suppressed in the dark day-bright night condition.
Postprandial plasma TG levels were higher when breakfast was consumed under bright light conditions.
Together, these findings suggest that the indoor light environment of insulin-resistant individuals may modulate metabolic variables as a function of time of day to influence long-term metabolic health.
In conclusion , by performing detailed 24-h metabolic phenotyping using frequent blood sampling, continuous indirect calorimetry, and skin temperature assessment, we demonstrate that the timing of light exposure can influence postprandial substrate management, the Energy expenditure and thermoregulation of insulin-resistant individuals.
In contrast to the Dim Day-Bright Evening condition, the Bright Day-Dim Evening condition had mostly favorable results: lower plasma glucose levels before dinner, greater energy expenditure in response to dinner.
The finding that postprandial TG levels after breakfast and postprandial glucose levels after dinner increased less in the dark day-bright night condition warrants further investigation. In the future, more research is required to exploit different lighting regimes in office buildings and home environments for their potential to prevent metabolic diseases.
Registration : ClinicalTrials.gov NCT03829982.
Funding : We acknowledge financial support from the Netherlands Cardiovascular Research Initiative – an initiative supported by the Dutch Heart Foundation (CVON2014–02 ENERGISE).
















