Long-term complications of type 2 diabetes mellitus, including microvascular and cardiovascular diseases, account for the majority of illness, death, and costs associated with this condition. Clinical trials have demonstrated a benefit of reducing chronic hyperglycemia on specific microvascular complications of diabetes. Additionally, trials have shown that some new classes of glucose-lowering medications have beneficial effects with respect to cardiovascular disease and kidney disease, primarily in participants with preexisting conditions, while other medications have been shown to have neutral effects or potentially side effects. harmful.
Background
Data are lacking on the comparative effectiveness of commonly used hypoglycemic medications, when added to metformin, with respect to microvascular and cardiovascular disease outcomes in people with type 2 diabetes.
Methods
We evaluated the comparative effectiveness of four commonly used hypoglycemic medications, added to metformin, in achieving and maintaining a glycated hemoglobin level less than 7.0% in participants with type 2 diabetes. Randomly assigned therapies were insulin glargine U-100 ( hereinafter glargine), glimepiride, liraglutide and sitagliptin.
Prespecified secondary outcomes regarding microvascular and cardiovascular disease included hypertension and dyslipidemia, confirmed moderately or severely increased albuminuria or an estimated glomerular filtration rate of less than 60 ml per minute per 1.73 m 2 of body surface area, peripheral neuropathy diabetes assessed with the Michigan Neuropathy Screening Instrument, cardiovascular events (major adverse cardiovascular events [MACE], hospitalization for heart failure, or an aggregate outcome of any cardiovascular event), and death.
Hazard ratios are presented with 95% confidence limits that are not adjusted for multiple comparisons.
Results
Over a median of 5.0 years of follow-up in 5047 participants, there were no material differences between the interventions with respect to the development of hypertension or dyslipidemia or with respect to microvascular outcomes; the mean overall rate (i.e., events per 100 participant-years) of moderately increased albuminuria levels was 2.6, of severely increased albuminuria levels was 1.1, of renal failure was 2.9, and of diabetic peripheral neuropathy of 16.7.
Treatment groups did not differ with respect to MACE (overall rate, 1.0), hospitalization for heart failure (0.4), death from cardiovascular causes (0.3), or all deaths (0.6).
There were small differences regarding the rates of any cardiovascular disease, with 1.9, 1.9, 1.4 and 2.0 in the glargine, glimepiride, liraglutide and sitagliptin groups, respectively.
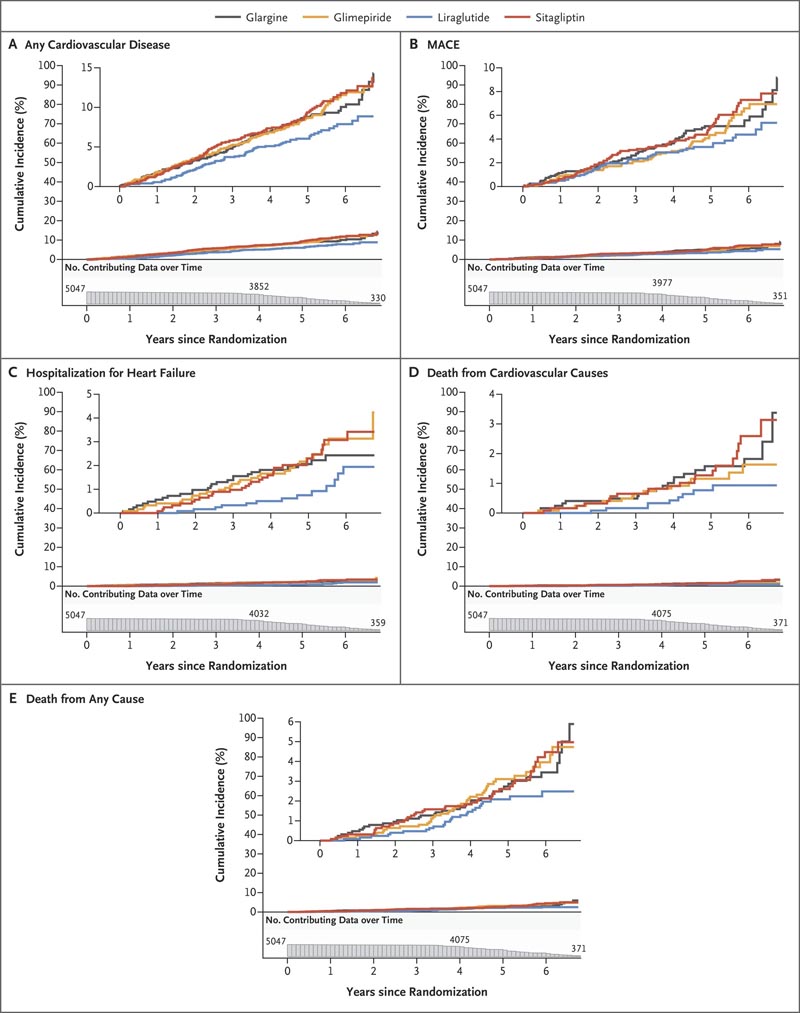
Figure : Cumulative incidences of cardiovascular outcomes and mortality in intention-to-treat analyses . Cumulative incidences of any cardiovascular disease (Panel A), a major adverse cardiovascular event (MACE) (Panel B), hospitalization for heart failure (Panel C), death from cardiovascular causes (Panel D), and death from any cause are shown. (Panel E) over 6.5 years of follow-up. The numbers plotted below the x-axis of each panel are the participants at risk for the outcome at each follow-up time point (i.e., the number of participants in whom a specific outcome event had not developed at that time point).
Conclusions In participants with type 2 diabetes, the incidences of microvascular complications and death were not significantly different among the four treatment groups. The findings indicated possible differences between the groups in the incidence of any cardiovascular disease. |
Discussion
The current trial showed significant differences between the four randomized treatments, when added to metformin, in the ability to achieve and maintain target glycated hemoglobin levels. Here, we evaluated secondary outcomes, including the differential effects of these agents with respect to microvascular and cardiovascular disease and their risk factors.
Most participants had hypertension or dyslipidemia at baseline, which is typical of a population with type 2 diabetes mellitus. More than 90% of participants who did not have hypertension or dyslipidemia at baseline were later classified as having these conditions, largely because their own care providers had started the medications. The only difference in the development of these conditions at 1 and 4 years of follow-up was that liraglutide may have had a relative benefit with respect to measured blood pressure. The glargine group may have had more incident hypertension due to the effect of insulin on sodium reabsorption in the kidney.
Despite differences between treatment groups in glycemia and hypertension, which are long-recognized risk factors for microvascular complications, there were no material differences in any of the microvascular complications that were evaluated. The absence of the expected effect of lower glycemia on microvascular complications has been observed in some trials, including diabetes prevention studies, and this absence has been attributed to inadequate separation of glycemic levels over time, duration insufficient assay, threshold effects or inadequate power. Any or all of these factors, including the small gap in glycemia, could have been operative in our trial.
The current trial used a comparative effectiveness approach to examine four different glucose-lowering medications. The different effects of these agents on microvascular complications, cardiovascular risk factors, and cardiovascular outcomes should be considered along with their glycemic effects when choosing therapies for type 2 diabetes. In this trial involving participants with type 2 diabetes of generally duration Briefly, the incidences of microvascular complications and death were not significantly different between the four treatment groups. The findings supported possible differences between treatment groups in the incidence of any cardiovascular disease.
(Funded by the National Institute of Diabetes and Digestive and Kidney Diseases and others; ClinicalTrials.gov GRADE number, NCT01794143. opens in new tab.)