A collaborative research effort by scientists from the UNC School of Medicine’s Departments of Radiology, Neurology, and Psychology and Neuroscience has demonstrated the deleterious effects of chronic cocaine use on functional networks in the brain.
Their study, titled "Changes in network connectivity after long-term cocaine use and abstinence ," was highlighted by the editor of the Journal of Neuroscience . The findings show that continued cocaine use affects the way crucial neural networks communicate with each other in the brain, including the default mode network (DMN), salience network (SN), and lateral cortical network (LCN). ).
"Disrupted communication between DMN and SN may make it harder to concentrate, control impulses, or feel motivated without the drug," said Li-Ming Hsu, PhD, assistant professor of radiology and senior author of the study. "Basically, these changes can affect how you respond to everyday situations, making recovery and resistance to cravings more challenging."
Hsu led this project during his postdoctoral stay at the Animal Magnetic Resonance Center in the Biomedical Research Imaging Center and the Department of Neurology. The work provides new insights into the brain processes underlying cocaine addiction and creates opportunities for the development of therapeutic approaches and the identification of an imaging marker for cocaine use disorders.
The brain functions like an orchestra , where each instrumentalist has a special role crucial to creating a coherent piece of music. Specific parts of the brain need to work together to complete a task. The LCN is active during dreams and reflections, the SN is crucial for attention, and the LCN, like a musical director, plays a role in our decision-making and problem-solving.
The research was motivated by observations from human functional brain imaging studies suggesting that chronic cocaine use alters connectivity within and between major brain networks. The researchers needed a longitudinal animal model to understand the relationship between brain connectivity and the development of cocaine dependence, as well as changes during withdrawal.
The researchers used a rat model to mimic human addiction patterns, allowing the models to self-dose with a touch to the nose. Coupled with advanced neuroimaging techniques, the behavioral approach allows for a deeper understanding of the brain’s adaptation to long-term drug use and highlights how addictive substances can alter the functioning of critical brain networks.
Hsu’s research team used functional MRI to explore changes in brain network dynamics in models self-administering cocaine. During a 10-day period followed by abstinence, the researchers observed significant alterations in network communication, particularly between DMN and SN.
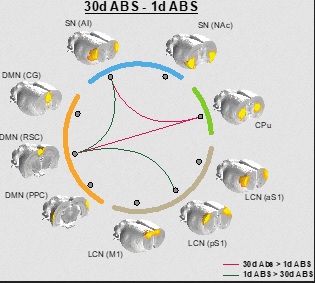
Image: This image illustrates changes in brain communication in rats after periods of cocaine abstinence. The lines between the images indicate greater or lesser connectivity after 1 day and 30 days without the drug; red lines represent areas with more connectivity and green lines show less connectivity over time.
These changes were more pronounced with higher cocaine intake during the 10 days of self-administration, suggesting a potential target for reducing cocaine cravings and helping those in recovery. Changes in the communication of these networks could also serve as useful imaging biomarkers for cocaine addiction.
The study also offered new insights into the anterior insular cortex (AI) and retrosplenial cortex (RSC). The first is responsible for emotional and social processing; while the latter controls episodic memory, navigation and imagination of future events. The researchers observed that there was a difference in coactivity between these two regions before and after cocaine ingestion. This circuit could be a potential target to modulate the behavioral changes associated with cocaine use disorders.
“Previous studies have demonstrated changes in functional connectivity with cocaine exposure; However, detailed longitudinal analysis of specific brain network changes, especially between the anterior insular cortex (AI) and the retrosplenial cortex (RSC), before and after cocaine self-administration and after prolonged abstinence, provides new knowledge,” Hsu said.