Secondary analysis of a randomized clinical trial
Aspirin is an antiplatelet agent that has been used at low doses (75-100 mg/d) for the prevention of cardiovascular events . Despite some recent unfavorable findings, it continues to be widely used for primary and secondary stroke prevention . Its main adverse effect is a greater tendency to bleeding.
Information on the effectiveness of low-dose aspirin in the primary prevention of stroke is derived from meta-analyses and results from recent large trials, most of which have been conducted in populations with mean ages less than 70 years. Despite some inconsistencies, these reports suggest a trend toward reduced ischemic stroke offset by increased intracerebral and other intracranial hemorrhage, with little overall impact on overall stroke incidence.
Clinical characteristics of older people include an inherent increased susceptibility to bleeding, which may be associated with increased fragility of small blood vessels. Additionally, older people experience a greater susceptibility to major and minor trauma as a result of falls and other accidents. Together, these results may alter the balance of risks and benefits of an antiplatelet drug, especially if given to low-risk individuals in a primary prevention setting . This concern is relevant given the high risk of stroke in older people, the global increase in older populations, and the importance of evaluating preventive strategies in this age group.
The Aspirin in Reducing Events in the Elderly (ASPREE) trial is the largest randomized controlled trial of low-dose aspirin focused on investigating the balance of risks and benefits of this therapy in an older age group. In addition to its large size, the ASPREE study design included independent adjudication of stroke and bleeding events by independent expert panels. Therefore, we were ideally positioned to evaluate the balance of risks and benefits of low-dose aspirin in a primary prevention setting . In this study, we provide a comprehensive report on the incidence of first stroke and bleeding events that occurred during the median 4.7 years of trial follow-up.
Importance
Low-dose aspirin has been widely used for primary and secondary prevention of stroke. The balance between potential reduction in ischemic stroke events and increased intracranial bleeding has not been established in older people.
Aim
To establish the risks of ischemic stroke and intracranial hemorrhage among healthy elderly patients receiving daily low-dose aspirin.
Design, environment and participants
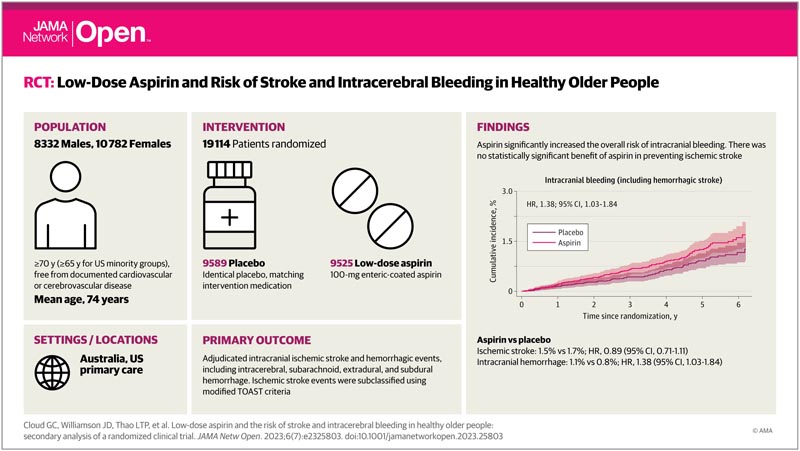
This secondary analysis of the randomized, double-blind, placebo-controlled Aspirin in Reducing Events in the Elderly (ASPREE) trial of daily low-dose aspirin was conducted among community-dwelling people in Australia or the US. Participants were older adults free of symptomatic cardiovascular disease. Recruitment occurred between 2010 and 2014, and participants were followed for a median (IQR) of 4.7 (3.6-5.7) years. This analysis was completed from August 2021 to March 2023.
Interventions
100 mg daily of enteric-coated aspirin or equivalent placebo.
Main results and measures
Stroke and stroke etiology were predetermined secondary outcomes and are presented with a focus on prevention of the initial stroke or intracranial hemorrhage event . The results were evaluated by reviewing the medical records.
Results
Among 19,114 older adults (10,782 women [56.4%]; median [IQR] age, 74 [71.6-77.7] years), 9,525 people received aspirin and 9,589 people received placebo.
Aspirin did not produce a statistically significant reduction in the incidence of ischemic stroke (hazard ratio [HR], 0.89; 95% CI, 0.71-1.11).
However, a statistically significant increase in intracranial bleeding was observed among individuals assigned to aspirin (108 individuals [1.1%]) compared with those receiving placebo (79 individuals [0.8%]; HR, 1 .38; 95% CI, 1.03-1.84).
This occurred due to an increase in a combination of subdural, extradural, and subarachnoid hemorrhage with aspirin compared with placebo (59 people [0.6%] vs. 41 people [0.4%]; HR, 1.45; 95% CI %, 0.98-2.16). Hemorrhagic stroke occurred in 49 individuals (0.5%) assigned to aspirin compared with 37 individuals (0.4%) in the placebo group (HR, 1.33; 95% CI, 0.87 -2.04).
Conclusions and relevance
This study found a significant increase in intracranial bleeding with daily low-dose aspirin, but no significant reduction in ischemic stroke. These findings may have particular relevance for older people prone to developing intracranial hemorrhage after head trauma.
Discussion
To our knowledge, the ASPREE randomized clinical trial is the first large-scale trial to study the risks and benefits of aspirin in an exclusively older primary prevention population , in which an increased bleeding tendency may alter the balance of risks and benefits. of aspirin. This is particularly relevant for intracerebral events because intracranial hemorrhage is typically less treatable than ischemic events and more often fatal or disabling . With previous trials of aspirin in mostly younger participants, an excess of intracerebral hemorrhagic events was frequently noted among individuals receiving active treatment, although the numbers were small and in most cases did not approach statistical significance. .
The primary finding of this secondary analysis of a randomized clinical trial was an increase in intracerebral hemorrhagic events, which in absolute terms outweighed a minor, nonsignificant reduction in ischemic strokes. Despite the older age of the cohort, the incidence of both types of events was low, with an overall rate of 5.8 per 1000 person-years of follow-up. The incidence of ischemic stroke was 0.5 incidents per 1000 person-years of follow-up lower, which was not statistically significant, while that of intracranial hemorrhage was 0.7 incidents higher, which was statistically significant.
These data expand previously reported findings on stroke outcomes in ASPREE by focusing on first stroke and bleeding events and by directly comparing the potential benefit of aspirin in preventing ischemic stroke with the risks of intracranial hemorrhage. The findings relate to a relatively healthy elderly population with extensive levels of blood pressure and lipid control and no pre-existing cardiovascular or cerebrovascular disease. No subgroups were identified in which the effect of aspirin was substantially different from the mean.
The lack of an effect of aspirin in reducing ischemic stroke was notable given the increased age-related risk in this population and the reported efficacy of aspirin in secondary stroke prevention. Of the strokes that occurred in the trial, 78.4% were adjudicated as ischemic in origin , and among etiologic subtypes, the largest differences between individuals assigned to aspirin or placebo were strokes caused by occlusion of small vessels (11 fewer cases) and those of suspected cardioembolic origin (9 fewer cases). However, there was little difference in ischemic strokes of large vessel origin in which an antiplatelet such as aspirin might be anticipated to be more effective. The lack of benefit in this subgroup may be due to chance with small numbers. However, the finding is consistent with the results of a randomized, placebo-controlled trial of aspirin (325 mg/d) in 372 people with asymptomatic internal carotid artery stenosis of 50% or more, which showed no evidence of benefit for 2 years. of follow up.
Among individuals randomized to aspirin, there were additional cases of intracerebral, subdural, and extradural hemorrhage , some occurring after trauma and others occurring spontaneously. No subgroup was identified in which the risk of bleeding was substantially different from the mean. Head trauma, usually as a result of falls, is common in older people, and additional cases of bleeding after such events are an important component of the risk-benefit equation of any antiplatelet agent in older adults. The majority of additional cases of intracerebral hemorrhage (8/12 events) occurred in the basal ganglia, where hypertensive arteriopathy is considered the dominant pathology. Surprisingly, fewer additional cases occurred in the lobar regions, where cerebral amyloid angiopathy is believed to be the predominant underlying pathology and is common in this age group.
Poor clinical outcomes after intracranial hemorrhagic events were reflected in higher mortality rates . Although intracerebral hemorrhage accounted for 21.6% of strokes, one-third of these (29/86 strokes) were fatal compared with 7.7% of ischemic strokes (24/312 strokes). The mortality rate after subarachnoid hemorrhage and subdural hematomas was similar among individuals randomized to treatment with aspirin or placebo.
The lack of benefit and potential risks of aspirin in primary stroke prevention provide additional evidence supporting the USPSTF’s recently published draft recommendation against routine prescribing of low-dose aspirin as a primary prevention measure , especially in people greater. Clinicians should be aware that, among fall-prone older people, the risks of intracerebral hemorrhage with aspirin may be higher than appears in this trial. Our results are also cautious regarding the inclusion of aspirin in a polypill to prevent cardiovascular disease in healthy older adults. Newer antiplatelet therapies, such as clopidogrel, ticagrelor, or prasugrel, have not been studied in a primary prevention setting and should not yet be considered as alternatives to aspirin for this indication.
ISRCTN.org Test Registration Identifier: ISRCTN83772183