Coronavirus disease 2019 (Covid-19), the disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was first identified in December 2019 and rapidly progressed to become a global pandemic.
Older people and those with coexisting conditions such as obesity, cardiovascular disease and diabetes mellitus are at higher risk of hospitalization or death from Covid-19. Some treatments such as monoclonal antibodies reduce the risk of progression of Covid-19. Additional therapeutic options could benefit patients and ease the burden on healthcare systems.
Remdesivir is a direct-acting nucleotide prodrug inhibitor of SARS-CoV-2 RNA-dependent RNA polymerase; has potent nanomolar activity in primary human airway epithelial cells. A phase 3 trial of remdesivir showed that both a 10-day course and a 5-day course of remdesivir shortened recovery time in hospitalized Covid-19 patients.
Early treatment of other acute viral infections improves clinical outcomes and reduces mortality, and this strategy has also been proposed for Covid-19. We hypothesized that earlier initiation of a short course of remdesivir treatment in outpatient settings would reduce hospitalizations and mortality.
Here, we report the results of a double-blind, randomized, placebo-controlled trial that evaluated the efficacy and safety of a 3-day course of remdesivir in high-risk non-hospitalized patients with Covid-19.
Background
Remdesivir improves clinical outcomes in hospitalized patients with moderate to severe coronavirus disease 2019 (Covid-19). It is not known whether the use of remdesivir in symptomatic non-hospitalized patients with Covid-19 who are at high risk of disease progression prevents hospitalization.
Methods
We conducted a randomized, double-blind, placebo-controlled trial in non-hospitalized patients with Covid-19 who had symptom onset in the previous 7 days and who had at least one risk factor for disease progression (age ≥60 years, obesity or certain coexisting medical conditions).
Patients were randomly assigned to receive intravenous remdesivir (200 mg on day 1 and 100 mg on days 2 and 3) or placebo.
The primary efficacy endpoint was a composite of hospitalization or death from any COVID-19-related cause on day 28. The primary safety endpoint was any adverse event. A secondary endpoint was a composite of a Covid-19-related medical visit or death from any cause by day 28.
Results
A total of 562 patients who were randomized and received at least one dose of remdesivir or placebo were included in the analyses: 279 patients in the remdesivir group and 283 in the placebo group.
The mean age was 50 years, 47.9% of patients were women, and 41.8% were Hispanic or Latino. The most common coexisting conditions were diabetes mellitus (61.6%), obesity (55.2%), and hypertension (47.7%).
COVID-19-related hospitalizations or deaths from any cause occurred in 2 patients (0.7%) in the remdesivir group and 15 (5.3%) in the placebo group (hazard ratio, 0.13 ; 95% confidence interval [CI], 0.03 to 0.59; P=0.008).
A total of 4 of 246 patients (1.6%) in the remdesivir group and 21 of 252 (8.3%) in the placebo group had a Covid-19-related medical visit on day 28 (hazard ratio, 0.19; 95% CI, 0.07 to 0.56).
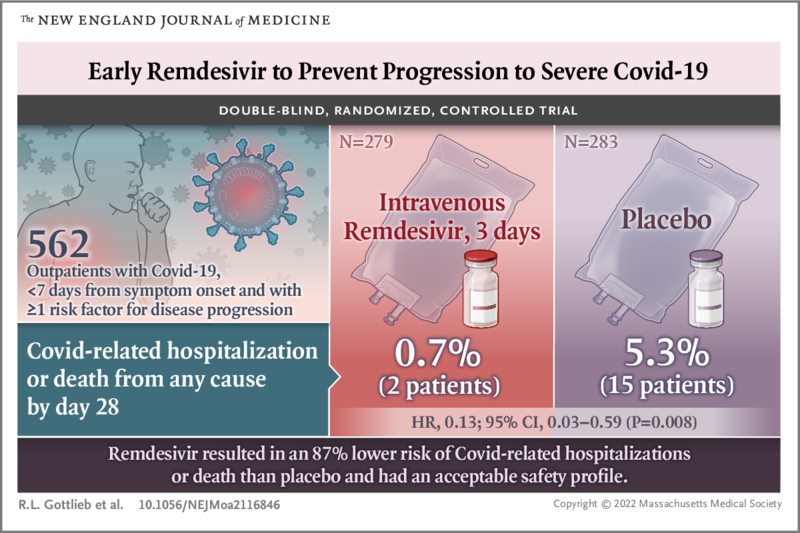
Conclusions Among non-hospitalized patients who were at high risk of Covid-19 progression, a 3-day course of remdesivir had an acceptable safety profile and resulted in an 87% lower risk of hospitalization or death than placebo. |
(Funded by Gilead Sciences; PINETREE ClinicalTrials.gov number, NCT04501952. opens in new tab; EudraCT Number, 2020-003510-12. opens in new tab.)