Background
Type 2 diabetes mellitus (T2DM) is known to be associated with neurobiological and cognitive deficits; however, its extent, its overlap with the effects of aging, and the effectiveness of existing treatments in the context of the brain are currently unknown.
Methods:
We characterized neurocognitive effects independently associated with T2DM and age in a large cohort of human subjects from the UK Biobank with cross-sectional neuroimaging and cognitive data. We then proceeded to assess the degree of overlap between T2DM- and age-related effects by applying correlation measures to the separately characterized neurocognitive changes.
Our findings were complemented by meta-analysis of published reports with cognitive or neuroimaging measures for T2DM and healthy controls (HC). We also evaluated in a cohort of people diagnosed with T2DM using the UK Biobank how chronicity of the disease and metformin treatment interact with the identified neurocognitive effects.
Results:
The UK Biobank dataset included cognitive and neuroimaging data (N = 20,314), including 1012 T2DM and 19,302 HC, aged 50 to 80 years .
The duration of T2DM ranged from 0 to 31 years (mean 8.5 ± 6.1 years); 498 were treated with metformin alone, while 352 received no medication. Our meta-analysis evaluated 34 cognitive studies (N = 22,231) and 60 neuroimaging studies: 30 from T2DM (N = 866) and 30 from aging (N = 1,088).
Compared with age, sex, education, and hypertension-matched HC, T2DM was associated with marked cognitive deficits , particularly in executive functioning and processing speed.
Likewise, we found that the diagnosis of T2DM was significantly associated with gray matter atrophy , mainly in the ventral striatum, cerebellum and putamen, with a reorganization of brain activity (decrease in the caudate and premotor cortex and increase in the subgenual area, orbitofrontal cortex), brainstem and posterior cingulate cortex).
The structural and functional changes associated with T2DM show marked overlap with effects that correlate with age, but appear earlier , and disease duration is associated with more severe neurodegeneration . Metformin treatment status was not associated with better neurocognitive outcomes.
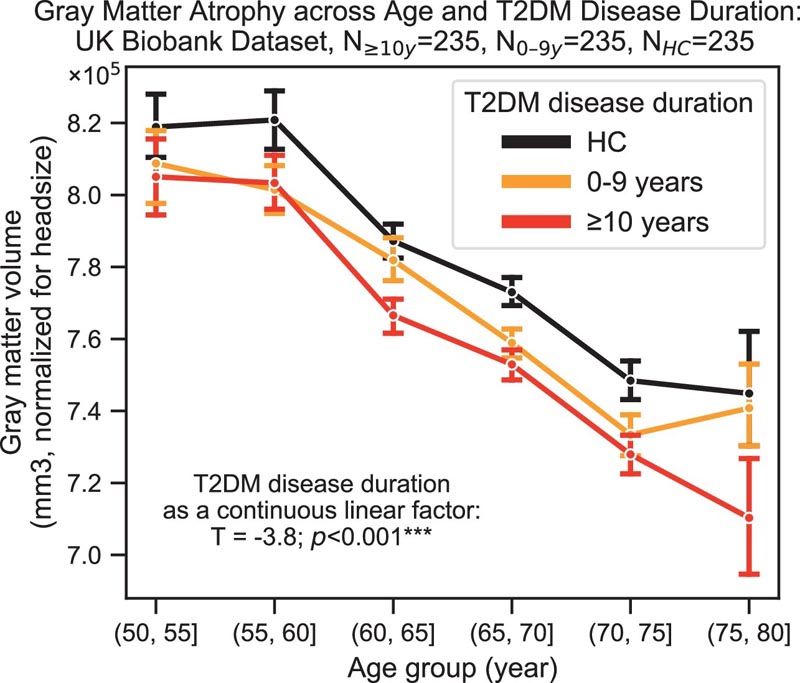
The progression of type 2 diabetes mellitus (T2DM) is significantly associated with gray matter atrophy, which accelerates the neurodegenerative effects observed in brain aging.
Conclusions: The neurocognitive impact of T2DM suggests a marked acceleration of normal brain aging. Gray matter atrophy in T2DM occurred approximately 26% ± 14% faster than that seen with normal aging; disease duration was associated with increased neurodegeneration. Mechanistically, our results suggest a neurometabolic component in brain aging. Clinically, neuroimaging-based biomarkers may provide a valuable complementary measure of T2DM progression and treatment efficacy based on neurological effects. |
Discussion
The UK Biobank dataset confirms that patients with T2DM show deficits in cognitive performance compared to healthy controls (HCs), even after controlling for age, sex, education and hypertension. These findings were supported by a meta-analysis of the published literature.
Deficits in cognitive performance were accompanied by marked brain atrophy in the T2DM sample compared to age-matched healthy controls.
Atrophy was most severe (6.2% gray matter loss compared to HC) in the ventral striatum, a region critical for learning, decision making, goal-directed behavior, and cognitive control. These cognitive functions, known collectively as executive functions, were (along with processing speed) also the most affected by T2DM.
The severity of neurodegeneration for all regions increased with increasing disease duration. We detected qualitatively consistent results in women and men; however, men exhibited stronger effects relative to T2DM. This result is consistent with the well-established neuroprotective effects of female hormones such as estrogen.
This result also suggests that the observed neurological effects of T2DM are the result of chronic degenerative processes that, for our female participants, may have at least partially improved before menopause.
Our findings indicate that structural brain imaging, in particular, may provide a clinically valuable metric for identifying and monitoring neurocognitive effects associated with T2DM.
Consistent with findings from previous studies that focused on brain and energy metabolism (Sokoloff, 1955; Clark, 1999), our results suggest that T2DM and its progression may accelerate pathways associated with typical brain aging. As T2DM decreases glucose availability in the brain, this chronic loss of energy can compromise brain structure and function.
We consider the possibility that, by the time T2DM is formally diagnosed, neuronal insulin resistance has already caused significant damage.
As such, our findings underscore the need for additional research on brain biomarkers for T2DM and treatment strategies that specifically target its neurocognitive effects.
Comments
Type 2 diabetes accelerates brain aging and cognitive decline
Analysis of UK Biobank data from 20,000 people shows that diabetes accelerates the normal brain aging process, with longer duration of diabetes linked to greater neurodegeneration
Scientists have shown that normal brain aging is accelerated by about 26% in people with progressive type 2 diabetes compared to people without the disease, reports a study published in eLife .
The authors evaluated the relationship between typical brain aging and that observed in type 2 diabetes, and observed that type 2 diabetes follows a pattern of neurodegeneration similar to that of aging, but progresses more rapidly. An important implication of this finding is that even typical brain aging may reflect changes in brain glucose regulation by insulin.
The results further suggest that by the time type 2 diabetes is formally diagnosed, there may already be significant structural damage to the brain.
Therefore, sensitive ways to detect changes in the brain associated with diabetes are urgently needed.
There is already strong evidence linking type 2 diabetes to cognitive decline, but few patients currently undergo comprehensive cognitive evaluation as part of their clinical care. It can be difficult to distinguish between normal brain aging that begins in middle age and brain aging caused or accelerated by diabetes. To date, no studies have directly compared neurological changes in healthy people over the lifespan with the changes experienced by people of the same age with diabetes.
"Routine clinical evaluations to diagnose diabetes typically focus on blood glucose , insulin levels, and body mass percentage," says first author Botond Antal, a doctoral student in the University’s Department of Biomedical Engineering. from Stony Brook, New York, USA. “However, the neurological effects of type 2 diabetes can reveal themselves many years before they can be detected by standard measures , so by the time conventional tests diagnose type 2 diabetes 2, patients may have already suffered irreversible brain damage.”
To define the impact of diabetes on the brain beyond normal aging, the team used the largest brain structure and function data set available across the human lifespan: UK Biobank data from 20,000 people from 50 at 80 years old.
This data set includes brain scans and measurements of brain function and contains data from both healthy people and people with a diagnosis of type 2 diabetes. They used this to determine which brain and cognitive changes are specific to diabetes, rather than just aging, and then confirmed these results by comparing them with a meta-analysis of nearly 100 other studies.
Their analysis showed that both aging and type 2 diabetes cause changes in executive functions, such as working memory, flexible learning and thinking, and changes in the brain’s processing speed.
However, people with diabetes had an additional 13.1% decline in executive function beyond age-related effects, and their processing speed decreased an additional 6.7% compared to people with the same age without diabetes. Their meta-analysis of other studies also confirmed this finding: people with type 2 diabetes had consistently and markedly lower cognitive performance compared to healthy people who were the same age and with similar education.
The team also compared brain structure and activity between people with and without diabetes using MRI scans. Here, they found a decline in the brain’s gray matter with age, primarily in a region called the ventral striatum, which is critical for the brain’s executive functions.
However, people with diabetes had even more pronounced declines in gray matter beyond the typical age-related effects: an additional 6.2% decrease in gray matter in the ventral striatum, but also loss of gray matter gray in other regions, compared to normal aging.
Together, the results suggest that the patterns of type 2 diabetes-related neurodegeneration strongly overlap with those of normal aging, but that neurodegeneration is accelerated. Furthermore, these effects on brain function were more severe with increasing duration of diabetes. In fact, diabetes progression was linked to a 26% acceleration of brain aging.
"Our findings suggest that type 2 diabetes and its progression may be associated with accelerated brain aging, possibly due to compromised energy availability causing significant changes in brain structure and function," concludes lead author Lilianne Mujica. Parodi, director of the Computational Neurodiagnostics Laboratory, Stony Brook University.
“By the time diabetes is formally diagnosed, this damage may have already occurred . But brain imaging could provide a clinically valuable metric for identifying and monitoring these neurocognitive effects associated with diabetes. “Our results underscore the need to investigate brain-based biomarkers for type 2 diabetes and treatment strategies that specifically target its neurocognitive effects.”
Final message This work emphasizes the role of diabetes in brain aging and cognitive functions which is a critical gap that needs to be filled due to the increasing trend in the prevalence of diabetes worldwide. Provides valuable information about specific brain regions altered during aging and diabetes. Furthermore, it reports how T2DM accelerates the decline in cognition and brain function associated with aging. Extensive analysis of human data sets and comparison with published data from other researchers supports the conclusion of this study; However, certain diabetes interventions that do not rescue brain damage need further validation. |
















