UNIVERSITY OF SHEFFIELD
This article presents and explains the principle behind an electrochemical method to enhance the antimicrobial action of plasma-activated hydrogel therapy (PAHT) in wound decontamination.
The same method also boosts the production of other molecules, such as reactive nitrogen species (RNS). Electrochemically enhanced PAHT is highly effective in eradicating common wound pathogens, Escherichia coli and Pseudomonas aeruginosa, and somewhat effective against Staphylococcus aureus. Overall, this study shows that the new PAHT dressing offers a promising alternative to antibiotics and silver-based dressings for controlling infection and stimulating wound healing.
Chronic wound infections are a silent pandemic, threatening to become a global health crisis. The treatment cost for chronic wounds like diabetic foot ulcers (DFUs) is estimated at $17 billion per year, surpassing the cost of the five most expensive cancers in the U.S. The challenge is global, and current predictions indicate that by 2030 there will be 578 million people with diabetes (a 36% increase from 2020), with over 30% of people with diabetes experiencing an ulcer during their lifetime, and 60% of these ulcers becoming infected. This growing problem is fueled by antimicrobial resistance (AMR), which is expected to cause 10 million deaths annually by 2050, with a potential $100 trillion shock to the global economy. Increased antibiotic use could push AMR out of control, escalating antibiotic contamination, damaging the environment, and creating large environmental reservoirs of antimicrobial resistance.
Unfortunately, chronic wound treatment mostly depends on a limited range of options. These include low-tech manual debridement and low-cost protective dressings or, alternatively, high-tech, high-cost antimicrobial dressings and medications. All address only one aspect of the problem. Severe infections require systemic antibiotics and amputation when all else fails.
Low-temperature atmospheric pressure plasma is emerging as a new and exciting technology to control infections and promote healing in hard-to-heal wounds. Plasma’s benefit has been demonstrated in clinical trials, showing that treatment reduces bacterial load in wounds and promotes healing without long-term adverse side effects. Plasma is thus unique in wound treatment due to its dual action in controlling infection and stimulating healing. This is primarily due to the unique and complex mix of reactive oxygen species (ROS) and reactive nitrogen species (RNS), collectively known as reactive oxygen and nitrogen species (RONS), generated by plasma.
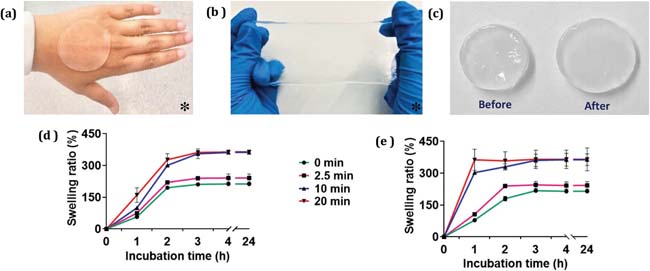
Figure: Physical characteristics of PVA hydrogel, including a) conformability, b) stretchability, and c) swelling before and after incubation in water. Quantified swelling ratios are also shown for untreated PVA hydrogels (0 min) and plasma-treated PVA hydrogels in d) floating potential (PVA-FLT) and e) grounded (PVA-GND) conditions, with plasma treatment times of 2.5, 10, and 20 min (n = 3). * Larger sections of PVA hydrogels are shown in (a) and (b) than used in experiments for clarity.
The treatment involves plasma (electric gas) activation of hydrogel dressings to produce a unique blend of various chemical oxidants that, when applied to the wound, effectively decontaminate and aid in the healing of chronic wounds.
The new method represents a major breakthrough that could revolutionize the treatment of ulcers and internal wounds, especially diabetic foot ulcers. Over 540 million people worldwide live with diabetes, of whom 30 percent will develop a foot ulcer during their lifetime.
An international team of scientists has developed an effective treatment for chronic wounds that uses no antibiotics but rather ionized gas to activate a dressing. The treatment involves plasma activation of hydrogel dressings (commonly used in wound care) with a unique blend of different chemical oxidants, effective for decontaminating and aiding in the healing of chronic wounds.
Researchers from the University of Sheffield and the University of South Australia, who led the study, believe this new method is a significant advance in combating antibiotic-resistant pathogens and has the potential to change the treatment of diabetic foot ulcers and internal wounds.
Professor Rob Short, Professor of Chemistry at the University of Sheffield and co-author of the study, said: "More than 540 million people live with diabetes worldwide, of whom 30 percent will develop a foot ulcer during their lifetime. This is a neglected global pandemic that will increase further in coming years due to rising obesity and lack of exercise."
"In England alone, between 60,000 and 75,000 people receive treatment each week for diabetic foot ulcers. Infection is one of the main risks. Increasingly, many infections do not respond to standard antibiotic treatment due to resistant bacteria, leading to 7,000 amputations a year."
"There is an urgent need for innovation in wound management and treatment, and it is a true privilege to be part of the international team that has been working on this alternative treatment for over 10 years."
The benefits of cold plasma ionized gas have already been demonstrated in clinical trials, showing that it not only controls infection but also promotes healing. This is due to the powerful chemical cocktail of oxidants, specifically reactive oxygen and nitrogen species (RONS), produced when oxygen and nitrogen molecules in the ambient air are mixed and activated.
Dr. Endre Szili, from the University of South Australia, who led the study, published this week in the journal Advanced Functional Materials, said, “Antibiotics and silver dressings are commonly used to treat chronic wounds, but both have downsides.”
“The growing resistance to antibiotics is a global challenge, and there are also major concerns about silver-induced toxicity. In Europe, silver dressings are being phased out for this reason.”
The international team of scientists demonstrated that plasma-activated hydrogels with RONS make the gel much more potent, effectively killing common bacteria. Although the study focused on diabetic foot ulcers, the technology could be applied to all chronic wounds and internal infections.
“Despite recent encouraging results in using plasma-activated hydrogel therapy (PAHT), we face the challenge of loading hydrogels with sufficient concentrations of RONS necessary for clinical use. We have overcome this obstacle by employing a new electrochemical method that enhances hydrogel activation,” stated Dr. Szili.
In addition to killing common bacteria (E. coli and P. aeruginosa) that cause wound infections, researchers say plasma-activated hydrogels may also help activate the body’s immune system, potentially helping to fight infections. "Chronic wound infections are a silent pandemic that threatens to become a global health crisis," added Dr. Szili.
“A major advantage of our PAHT technology is that it can be used to treat all wounds. It is an environmentally safe treatment that uses natural components of air and water to produce its active ingredients, which break down into non-toxic, biocompatible components.”
“The active ingredients could be delivered over a prolonged period, improving treatment and with more potential to penetrate a tumor. Plasma has enormous potential in the medical world, and this is just the tip of the iceberg,” stated Dr. Szili.
The next step will involve clinical trials to optimize the electrochemical technology for human patient treatment.
An electrochemical approach was investigated and characterized to enhance the production of reactive oxygen and nitrogen species (RONS) in plasma-activated hydrogel (PAHT) for controlling the growth of common pathogenic bacteria found in wounds. Through electrochemical enhancement of the He plasma jet treatment system, an unprecedented amount of 3.4 mM H2O2 was produced in targeted PVA hydrogels. These dressings were highly effective in controlling the growth of E. coli and P. aeruginosa and slightly effective against S. aureus.
Next, in vivo validation of the new PAHT dressings will be necessary to ensure that the technology is effective and safe for wound treatment. Nevertheless, the new technology offers a promising alternative to existing antibiotic and silver dressings, which will need to be replaced due to the increasing problems of antimicrobial resistance and cumulative silver toxicity.















