A multicenter study led by Vanderbilt University Medical Center (VUMC) and the Lipscomb University College of Pharmacy in Nashville has identified a new potential treatment for acute heart failure, one of the leading causes of hospitalization and death.
The medication, dapagliflozin, was initially approved for the treatment of type 2 diabetes, but has since been shown to reduce the risk of hospitalization for heart failure and death in patients with serious health issues including chronic heart and kidney diseases and increased cardiovascular risk.
In a report this month in the Journal of the American College of Cardiology, researchers found that dapagliflozin also benefits patients after hospital admission for acute heart failure. The drug improves diuresis, the removal of excess fluid from the lungs, thereby alleviating congestion and potentially reducing hospital stays.
"We demonstrated the safety and efficacy of initiating dapagliflozin within the first day of hospitalization for acute heart failure," said the lead author of the article, Zachary Cox, PharmD, professor of Pharmacy Practice at Lipscomb University. This "will have an international impact on the treatment of acute heart failure."
Every year, 800,000 patients with acute heart failure are admitted to U.S. hospitals from emergency rooms. These patients are at high risk of prolonged hospital stays and death. The annual cost of treating acute heart failure in the United States is estimated to exceed $34 billion.
Diuretics are administered to most patients with acute heart failure to improve symptoms and pulmonary congestion caused by fluid buildup. However, the optimal approach to diuretic therapy in hospitalized patients with acute heart failure remains poorly defined and contributes to prolonged hospital stays and high rates of death and readmission.
Additionally, many patients do not respond to diuretics and approximately half of patients are discharged with persistent congestion. This can lead to patients returning to the hospital shortly after discharge and being readmitted for further heart failure treatment.
Dapagliflozin is a sodium-glucose cotransporter 2 (SGLT2) inhibitor that acts on the kidneys to increase the elimination of sodium and glucose from the body. In April 2020, VUMC initiated a randomized clinical trial of the drug in hospitalized patients with acute heart failure.
Despite the COVID-19 pandemic, which peaked midway through the study, researchers were able to enroll 240 patients and complete the trial, "thanks to diligent effort and collaboration between the CERI research team and... the emergency medicine and cardiology departments," said Cox.
Patients were enrolled at five sites in addition to VUMC: TriStar Centennial Medical Center and Ascension St. Thomas Hospital West in Nashville, the University of North Carolina in Chapel Hill, the University of Mississippi Medical Center in Jackson, and INTEGRIS Health Baptist Medical Center in Oklahoma City.
Within 24 hours of admission for acute heart failure, patients were randomized to receive dapagliflozin or conventional diuretic therapy.
While early administration of dapagliflozin did not improve weight-based diuretic efficiency compared to conventional treatment, patients who received the drug experienced no increase in adverse events, required shorter periods of intravenous diuresis, and were discharged faster during the five-day study period.
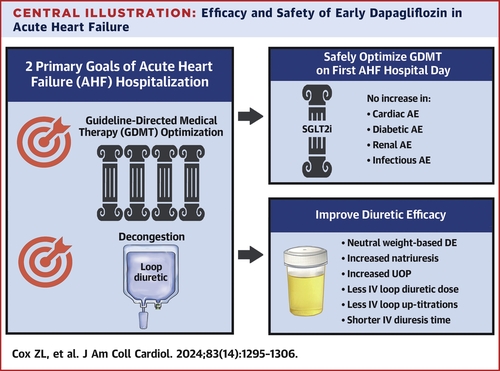
The trial demonstrated the safety and efficacy of initiating medication during early hospitalization that will continue to be prescribed at discharge to help achieve optimal outpatient therapy and reduce the likelihood of readmission.
"It’s a way to improve diuresis and to begin implementing guideline-directed medical therapy in patients with acute heart failure," said Lindenfeld.
The study was designed by JoAnn Lindenfeld, MD, and Sean Collins, MD, MSc, of VUMC, and by Cox, a member of the VUMC heart failure research team. Lindenfeld, a professor of Medicine in the Division of Cardiology, is nationally known for her innovative contributions to the field of heart failure.
Conclusions
- Early dapagliflozin during hospitalization for acute heart failure (AHF) is safe and serves as a component of optimization for decongestion and guideline-directed medical therapy (GDMT).
- Dapagliflozin was not associated with a statistically significant reduction in weight-based diuretic efficacy, but it was associated with evidence of increased diuresis among AHF patients.
Collins, a professor of Emergency Medicine, directs the Center for Emergency Care Research and Innovation, a national leader in emergency care research, co-directs the Vanderbilt Coordinating Center, which supports VUMC-led clinical research, and serves as associate director of emergency medicine and trial research at the Vanderbilt Institute for Medicine and Public Health. Cox is a member of the Heart Failure Society of America and has extensively published in this field.
Other VUMC co-authors include Cathy Jenkins, MS, and Frank Harrell Jr., PhD, from the Department of Biostatistics, and Christina Kampe, MAcc, Karen Miller, RN, MPA, and William Stubblefield, MD, MPH, from the Department of Emergency Medicine.
The study was an investigator-initiated trial funded by AstraZeneca but conducted independently by VUMC researchers. Dapagliflozin is marketed under the brand FARXIGA. Acute heart failure research at VUMC is partially supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health.