Early widely available outpatient treatments for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection are still needed to prevent severe coronavirus disease 2019 (Covid-19) due to decreased vaccine efficacy and the appearance of new variants. Access to current outpatient therapies remains limited, serious drug interactions may preclude their use, and the efficacy of monoclonal antibodies is challenged by viral evolution.
Drugs that meet this need may include metformin, ivermectin, and fluvoxamine based on biophysical models, which predicted that protein translation may be a target of antiviral therapy.
Background
Early treatment to prevent severe coronavirus disease 2019 (Covid-19) is an important component of the comprehensive response to the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic.
Methods
In this phase 3, double-blind, randomized, placebo-controlled trial, we used a 2 by 3 factorial design to test the effectiveness of three repurposed drugs (metformin, ivermectin, and fluvoxamine) in preventing severe SARS-CoV-2 infection. 2 in non-hospitalized adults who had enrolled within 3 days of a confirmed diagnosis of infection and less than 7 days after symptom onset.
The patients were between 30 and 85 years old, and all were overweight or obese. The primary composite endpoint was hypoxemia (≤93% oxygen saturation on home oximetry), emergency department visit, hospitalization, or death.
All analyzes used controls who had undergone concurrent randomization and adjusted for SARS-CoV-2 vaccination and receipt of other trial medications.
Results
A total of 1431 patients were randomized; of these patients, 1323 were included in the primary analysis. The median age of the patients was 46 years; 56% were women (6% of whom were pregnant) and 52% had been vaccinated.
The adjusted odds ratio for a primary event was 0.84 (95% confidence interval [CI], 0.66 to 1.09; P = 0.19) with metformin, 1.05 (95% CI). , 0.76 to 1.45; P = 0.78) with ivermectin and 0.94 (95% CI, 0.66 to 1.36; P = 0.75) with fluvoxamine.
In prespecified secondary analyses, the adjusted odds ratio for emergency department visit, hospitalization, or death was 0.58 (95% CI, 0.35 to 0.94) with metformin, 1.39 (95% CI, 0.35 to 0.94). 95% CI, 0.72 to 2.69) with ivermectin and 1.17 (95% CI, 0.35 to 0.94) with CI, 0.57 to 2.40) with fluvoxamine. The adjusted odds ratio for hospitalization or death was 0.47 (95% CI, 0.20 to 1.11) with metformin, 0.73 (95% CI, 0.19 to 2.
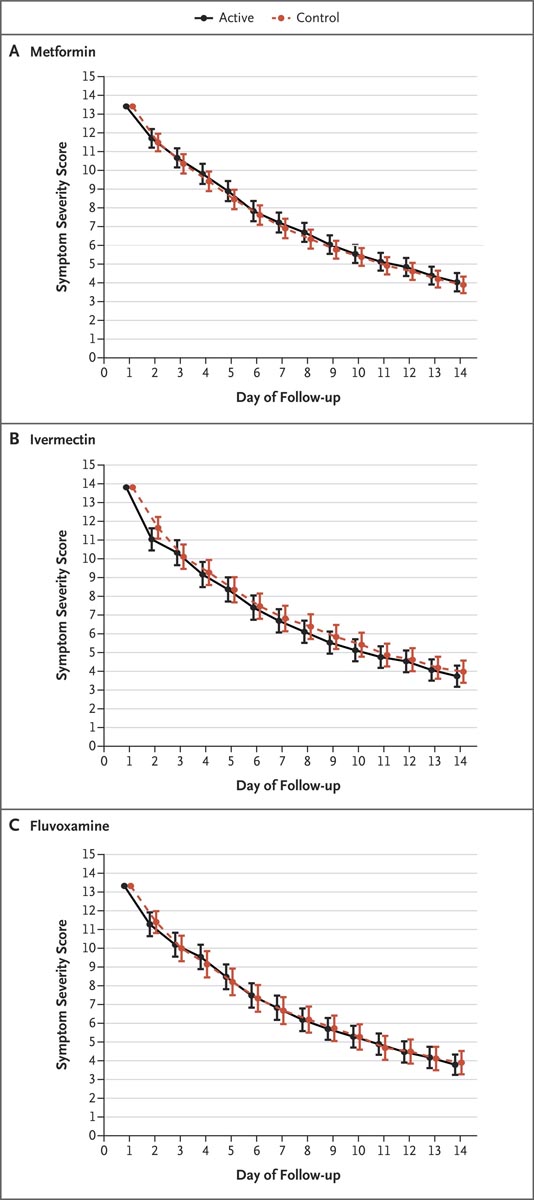
Figure : Total scores on a symptom severity scale over a 14-day period. The three panels present the composite symptom scores in the active treatment groups and the control groups among patients who received metformin, ivermectin, or fluvoxamine. Scores were calculated using a generalized estimating equation after adjusting for baseline score, vaccination status, and receipt of other medications during the trial. The y-axis shows the composite score of 14 symptoms, which were rated as none (0), mild (1), moderate (2), or severe (3). Overall, 80% of patients contributed data to symptom records; the frequency of missing data was approximately 25% on each of the 14 days.
Conclusions None of the three medications that were evaluated prevented the development of hypoxemia, a visit to the emergency department, hospitalization or death associated with Covid-19. |
Discussion
In this placebo-controlled clinical trial of metformin, ivermectin, and fluvoxamine for early outpatient treatment of SARS-CoV-2 infection, none of the three medications had a significant effect on the composite primary endpoint of hypoxemia, department visit of emergencies, hospitalization, or death.
A potential benefit in preventing the most serious components of the primary endpoint (emergency department visit, hospitalization, or death) was demonstrated for metformin. However, this finding was a prespecified secondary endpoint and therefore cannot be considered definitive pending the results of other trials.
In this randomized trial involving overweight and obese adults, none of the three trial drugs prevented a primary event of hypoxemia, emergency department visit, hospitalization, or death.
Analysis of a prespecified secondary outcome suggested a possible reduction in a composite endpoint of emergency department visit, hospitalization, or death with metformin.
None of the trial drugs resulted in less symptom severity than the identical placebo.
(Funded by Parsemus Foundation and others; COVID-OUT ClinicalTrials.gov number, NCT04510194. opens in new tab.)
















