
Research Highlights:
|
Background
Semaglutide, a glucagon-like peptide-1 receptor agonist, has been shown to reduce the risk of adverse cardiovascular events in patients with diabetes. It is unknown whether semaglutide can reduce the cardiovascular risk associated with overweight and obesity in the absence of diabetes.
Methods
In a multicenter, double-blind, randomized, placebo-controlled, event-based superiority trial, we enrolled patients 45 years of age or older who had preexisting cardiovascular disease and a body mass index (weight in kilograms divided by the square of height in meters) of 27 or more, but without a history of diabetes.
Patients were randomly assigned in a 1:1 ratio to receive once-weekly subcutaneous semaglutide at a dose of 2.4 mg or placebo.
The primary cardiovascular endpoint was a composite of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke in an analysis of time to first event. Safety was also evaluated.
Results
A total of 17,604 patients were enrolled ; 8,803 were assigned to receive semaglutide and 8,801 to receive placebo. The mean (±SD) duration of exposure to semaglutide or placebo was 34.2 ± 13.7 months, and the mean duration of follow-up was 39.8 ± 9.4 months.
A primary cardiovascular event occurred in 569 of 8803 patients (6.5%) in the semaglutide group and in 701 of 8801 patients (8.0%) in the placebo group (hazard ratio, 0.80; range 95% confidence, 0.72 to 0.90; P<0.001).
Adverse events leading to permanent discontinuation of the trial product occurred in 1461 patients (16.6%) in the semaglutide group and 718 patients (8.2%) in the placebo group (P<0.001).
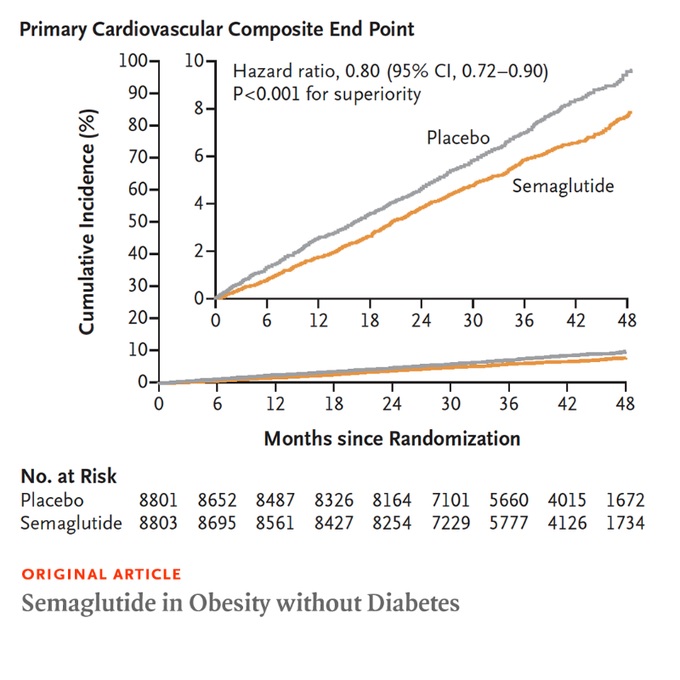
Conclusions In patients with preexisting cardiovascular disease and overweight or obesity but without diabetes, weekly subcutaneous semaglutide 2.4 mg was superior to placebo in reducing the incidence of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke. in a medium follow-up. 39.8 months. |
(Funded by Novo Nordisk; ClinicalTrials.gov, NCT03574597)
















