Background
Nitazoxanide is widely available and exerts broad-spectrum antiviral activity in vitro. However, there is no evidence of its impact on severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.
Methods
In a multicenter, randomized, double-blind, placebo-controlled trial, adult patients presenting up to 3 days after the onset of coronavirus disease 2019 (COVID-19) symptoms (dry cough, fever, and/or fatigue) were enrolled.
After confirmation of SARS-CoV-2 infection by reverse transcriptase PCR on a nasopharyngeal swab, patients were randomized 1:1 to receive nitazoxanide (500 mg) or placebo, three times daily, for 5 days.
The primary outcome was complete resolution of symptoms. Secondary outcomes were viral load, laboratory tests, serum biomarkers of inflammation, and hospitalization rate. Adverse events were also evaluated.
Results
From June 8 to August 20, 2020, 1,575 patients were examined. Of these, 392 were analyzed (198 placebo, 194 nitazoxanide). The median (interquartile range) time from symptom onset to first dose of study drug was 5 (4-5) days.
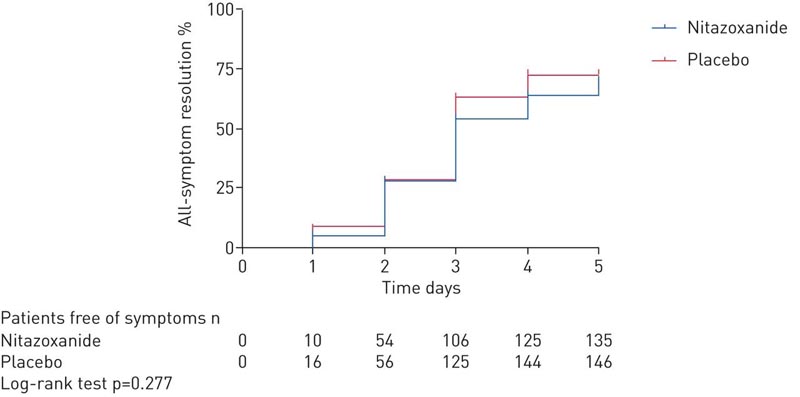
At the 5-day study visit, symptom resolution did not differ between the nitazoxanide and placebo arms.
Collected swabs were negative for SARS-CoV-2 in 29.9% of patients in the nitazoxanide group versus 18.2% in the placebo group (p = 0.009).
Viral load was reduced after nitazoxanide compared to placebo (p = 0.006).
The percentage reduction in viral load from baseline to the end of treatment was greater with nitazoxanide (55%) than with placebo (45%) (p = 0.013).
Other secondary outcomes were not significantly different. No serious adverse events were observed.
Discussion
In this multicenter, double-blind, randomized, placebo-controlled trial of patients with mild COVID-19, we found that resolution of symptoms (dry cough, fever, and fatigue) did not differ between nitazoxanide and placebo after 5 days of therapy .
Nitazoxanide was safe, significantly reduced viral load, and increased the proportion of patients who tested negative for SARS-CoV-2 after 5 days of therapy compared to placebo. Nitazoxanide did not prevent hospitalization or produce any changes in complete blood count, CRP levels, or serum biomarkers of inflammation. Additionally, nitazoxanide is inexpensive, widely available, and to date, other recommended therapies have not been shown to provide any benefit in this population.
There is an urgent need for evidence-based pharmacotherapeutic drugs for COVID-19. In the challenging context of a pandemic, drug repurposing can reduce the time-consuming drug development process and enable rapid deployment of effective therapies to the population.
Prior to the implementation of this trial, the National Institutes of Health Clinical Collection library, a collection of 727 U.S. Food and Drug Administration (FDA)-approved drugs or drug-like compounds with a history of use in human clinical trials, was examined for in vitro antiviral activity against SARS-CoV-2 (Supplementary Figure S1). Nitazoxanide and tizoxanide, its active metabolite, significantly reduced the viral load in Vero E6, human embryonic kidney (HEK 293T) and lung epithelial (Calu-3) cells infected with SARS-CoV-2, without inducing the loss of cell viability.
The adverse effect profile of nitazoxanide is well known, as it has been commercially available and used clinically since 1996; in fact, a commercial formulation was used in this trial. A dosing regimen of 500 mg every 12 h is approved and commonly prescribed for the treatment of intestinal parasitosis, with few adverse events reported.
In our study, the same dose was administered, but every 8 h, based on our group’s in vitro pharmacological studies and published data on plasma concentration, in order to maximize the potential inhibition of SARS-CoV-2 in vivo. We assumed that high ratios of the maximum serum concentration at safe doses in humans and the maximum effective antiviral concentration in vitro would translate into a greater potential to achieve viral suppression at approved doses.
Nitazoxanide failed to meet the primary outcome in patients with mild COVID-19 when evaluated after 5 days of treatment. Consistent with in vitro data, we observed significant reductions in viral load after a 5-day course of nitazoxanide in patients with mild COVID-19. This effect may have an epidemiological impact, potentially reducing community spread of SARS-CoV-2, morbidity and mortality.
Conclusions In patients with mild COVID-19, resolution of symptoms did not differ between the nitazoxanide and placebo groups after 5 days of treatment. However, early therapy with nitazoxanide was safe and significantly reduced viral load. |