Key points Ask Do people with generalized anxiety disorder (GAD) show abnormal physiological, perceptual, or neural responses during peripheral β-adrenergic stimulation that may indicate interoceptive dysfunction? Findings In this randomized crossover clinical trial, patients with GAD exhibited hypersensitivity to adrenergic stimulation, as well as increased interoceptive sensation and flattening of ventromedial prefrontal cortex activity compared to healthy participants. Meaning This study provides evidence for the dysfunctional contributions of the autonomic and central nervous system to the pathophysiology of GAD and suggests that the ventromedial prefrontal cortex may be a treatment target. Importance β-Adrenergic stimulation causes heart palpitations and dyspnea, key features of acute anxiety and sympathetic activation; However, no neuroimaging studies have examined how pharmacological modulation of interoceptive signals is associated with fear-related neurocircuitry in people with generalized anxiety disorder (GAD). |
Using an adrenaline-like challenge during brain imaging, we found that abnormal communication between the heart and brain contributes to increased fear in women with generalized anxiety disorder
Generalized anxiety disorder (GAD), the most common clinical manifestation of anxiety, is characterized by excessive, uncontrollable anxiety and worry that persists for at least 6 months.
Patients with GAD frequently show resistance to pharmacotherapy and psychotherapy, making it one of the most difficult anxiety disorders to treat.
Generalized anxiety disorder affects nearly twice as many women as men, and comorbidities with depression, substance use, and other anxiety disorders are common. People with GAD often experience symptoms of hyperarousal, including restlessness, feeling nervous or nervous, muscle tension, and insomnia.
Patients with GAD also seek cardiologic evaluation for symptoms of autonomic arousal at the same time as those with panic disorder, but such symptoms (e.g., fast heart rate [HR], shortness of breath, and sweating) do not consistently correlate with Peripheral autonomic indices in outpatient studies.
Consequently, their perception of physiological arousal often does not match their actual physiological state, suggesting that interoceptive dysfunction is a characteristic feature of the disorder. Identifying substrata of this dysfunction that are involved in disease-modifying processes could provide new targets for treatments that may help overcome high levels of anxious relapse and resistance to existing treatments.
Aim
Examine the neural circuitry underlying autonomic arousal induced through isoproterenol, a fast-acting peripheral β-adrenergic agonist similar to adrenaline.
Design, environment and participants
This randomized crossover clinical trial of 58 women with artifact-free data was conducted from January 1, 2017 to November 31, 2019 at the Laureate Institute for Brain Research in Tulsa, Oklahoma.
Exhibitions
Functional magnetic resonance imaging was used to evaluate neuronal responses during random intravenous bolus infusions of isoproterenol (0.5 and 2.0 μg) and saline, each administered twice in a double-blind manner.
Main results and measures
Whole-brain blood oxygen level-dependent responses during isoproterenol administration in GAD patients versus healthy comparators. Cardiac and respiratory responses, as well as interoceptive awareness and anxiety, were also measured during the infusion protocol.
Results
Of the 58 women participating in the study, 29 had GAD (mean [SD] age, 26.9 [6.8] years) and 29 were healthy matched comparators (mean [SD] age, 24.4 [5.0] years ).
During the 0.5 μg isoproterenol dose, the GAD group showed higher heart rate responses ( b = 5.34; 95% CI, 2.06-8.61; P = 0.002), higher intensity indices higher levels of cardiorespiratory sensations ( b = 8.38; 95% CI, 2.05-14.71; p = 0.01), higher levels of self-reported anxiety ( b = 1.04; 95% CI, 0.33-1.76; P = .005) and significant hypoactivation in the ventromedial prefrontal cortex (vmPFC) that was evident throughout the peak response (Cohen d = 1.55; P < .001) and early recovery periods (Cohen d = 1.52; P < .001).
Correlational analysis of physiological and subjective indices and percent signal change extracted during the 0.5 μg dose revealed that vmPFC hypoactivation was inversely correlated with heart rate ( r 56 = −0.51, adjusted P = 0.001) and the retrospective intensity of both heartbeat ( r 56 = −0.50, adjusted p = 0.002) and respiratory sensations ( r 56 = −0.44, adjusted p = 0.01).
Hypoactivation of the ventromedial prefrontal cortex was inversely correlated with continuous marking ratings at a trend level ( r 56 = −0.38, adjusted p = 0.051), while anxiety ( r 56 = −0.28, p adjusted = 0.27) and chronotropic dose 25 ( r 56 = −0.14, adjusted p = 0.72) did not show such an association.
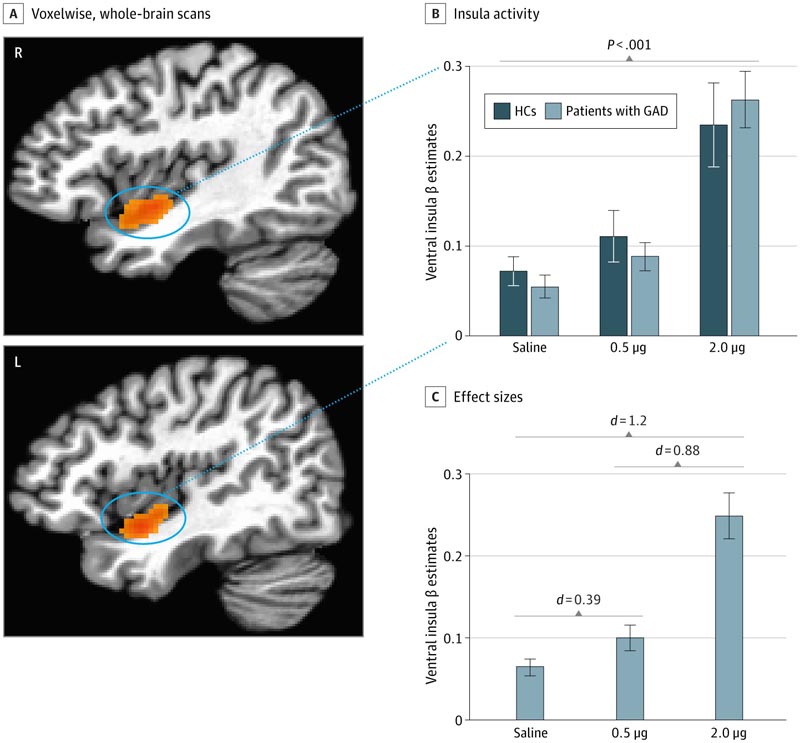
A, Increased activity localized within bilateral clusters of the ventral insular cortex was observed in all participants via voxel-wise whole-brain analysis during the time of peak effect of 2.0 isoproterenol infusion. 0 μg. B, No significant group differences were found within the ventral insular cortex groups at each dose. The 2.0 μg infusion elicited a significantly greater insula response than both saline and the 0.5 μg infusion. Clusters identified at 2.0 μg were used as masks to extract signal change for each dose, and saline infusions were based on previous studies 18 , 24 in which the 2.0 μg dose was shown to be sensitive in eliciting insula activity in healthy individuals. C, Cohen d effect sizes comparing each dose of isoproterenol with saline. Error bars indicate SEM. GAD indicates generalized anxiety disorder; HC, healthy comparator.
Discussion
We used peripheral β-adrenergic stimulation with isoproterenol to evaluate physiological, subjective, and neural responses during fMRI in women with GAD compared to healthy controls (HG). As expected, this manipulation caused dose-dependent increases in cardiorespiratory parameters in all participants.
However, the GAD group showed hypersensitivity to the lowest dose of isoproterenol in all response modalities. Specifically, during the peak window of the 0.5 μg dose, the GAD group demonstrated significant elevations in heart rate and also reported significantly stronger cardiorespiratory sensations during this dose and significantly more anxiety than HCs during both doses.
Whole-brain fMRI analyzes revealed significant differences between groups only during the 0.5 μg infusion, with the GAD group exhibiting bilateral vmPFC hypoactivation throughout the peak and early recovery epochs and hypoactivation of the parietal cortex. lower left during the peak period compared to the HC.
Of note, vmPFC activation differences were moderately to strongly correlated with HR and cardiorespiratory self-report during the 0.5 μg infusion. The lack of group differences in physiological or neural responses to saline or 2.0 μg infusion highlighted the sensitivity of the GAD group to sympathetic arousal signals during lower levels of adrenergic stimulation.
Conclusions and relevance
In this randomized crossover clinical trial, women with GAD exhibited autonomic hypersensitivity during low levels of adrenergic stimulation characterized by elevated heart rate, increased interoceptive awareness , increased anxiety, and a flattened neural response localized in the vmPFC.
These findings support the notion that autonomic hyperarousal may be associated with regulatory dysfunctions in the vmPFC, which could serve as a treatment target to help GAD patients more appropriately assess and regulate sympathetic activation signals.
















