Summary As our population ages, age-related pathologies are becoming more common. Deterioration of skeletal muscle and the immune system manifests as sarcopenia and immune senescence, respectively. The disease burden of these pathologies emphasizes the need for a better understanding of the underlying mechanisms. Skeletal muscle has become a powerful regulator of immune system function. As such, skeletal muscle could be the central integrator between sarcopenia and immune senescence in an aging biological system. Therapeutic approaches targeting skeletal muscle could restore function of both the muscle and the immune system. In this review, therefore, we describe the current, although still fragmentary, knowledge on the possible communication pathways of the muscle and the immune system. how they are affected by aging skeletal muscle and discuss possible treatment strategies. The review is intended to be hypothesis generating and should therefore stimulate further research in this important scientific field. |
Skeletal muscle as a potential central link between sarcopenia and immune senescence
Biological aging is defined by the loss of physiological integrity. Almost every organ in the human body is affected by the detrimental effects of aging. Skeletal muscle is no exception. Muscle mass and function decline with age. This age-dependent loss of muscle quality and quantity defines the sarcopenic phenotype according to the European Working Group on Sarcopenia in Older People .
Since 2018, sarcopenia is considered a muscle disease and muscle strength is superior to muscle mass in predicting adverse outcomes. Therefore, muscle strength is considered the main parameter defining sarcopenia.
The importance of a clinical definition that identifies sarcopenic patients is highlighted by an increasingly aging population. Currently, about 10% of elderly patients are considered sarcopenic. This number is expected to increase dramatically. In Europe, a 72% increase in the number of sarcopenic patients is expected until 2045, which will severely affect quality of life.
However, an exact understanding of the underlying mechanisms leading to sarcopenia and its clinical consequences is still lacking. Immune dysregulation and chronic inflammation have been discussed in the multifaceted pathogenesis of sarcopenia. The interaction between the immune system and the muscle compartment has been thought to be unilateral.
Skeletal muscle has been shown to regulate immune processes and the inflammatory response.
With respect to immune function, although it lacks an indisputable definition, the term immune senescence is commonly used to summarize age-dependent decline of the immune system. Key features of immune senescence are thymic atrophy, accumulation of senescent T cells, impaired function of innate immune cells such as NK cells, macrophages and neutrophils, and defective maintenance and functional response of lymphocytes
Age-dependent alterations in skeletal muscle immune function have also been observed. Therefore, sarcopenia and immune senescence could be linked/interact through skeletal muscle. The potential central role of skeletal muscle in regulating its own function and the immune system during aging is discussed.
The burden of sarcopenia: a risk factor for infections
Recently, several adverse outcomes of sarcopenia have been identified. These include, but are not limited to, an increased risk of falls leading to fractures, disability and functional impairment, dysphagia, lower quality of life, and all-cause mortality.
Sarcopenia predicts risk of infection after surgery. Furthermore, after three weeks of hospitalization, patients diagnosed with sarcopenia showed a two-fold increased risk of developing nosocomial infections. The impact of sarcopenia on the risk of infection in community-dwelling patients is less clear as the lack of epidemiological studies precludes a conclusive statement. However, sarcopenia predicts both the risk of community-acquired pneumonia in the elderly as well as 90-day mortality in patients with aspiration pneumonia.
Although the studies described above do not establish causality, they suggest a link between altered muscle function and an altered immune response to pathogens. Given the high incidence of sarcopenia and the increased risk of infections in elderly patients, the implications are profound, as sarcopenia could constitute both a clinical predictor for patients at risk and a possible therapeutic target to improve morbidity associated with infections in the elderly.
Skeletal muscle as a potential central regulator of immune system function
In the last two decades, the perception of skeletal muscle as a pure locomotor unit has changed. Muscle is increasingly recognized as an organ with immunoregulatory properties. As such, skeletal muscle cells modulate immune function by signaling through different soluble factors, cell surface molecules or cell-cell interactions. Although our knowledge of muscle-immune system interaction has advanced considerably, the impact of age is relatively unknown.
Sarcopenia may severely disrupt this interaction, providing a possible explanation for the clinical outcomes observed in patients with sarcopenia.
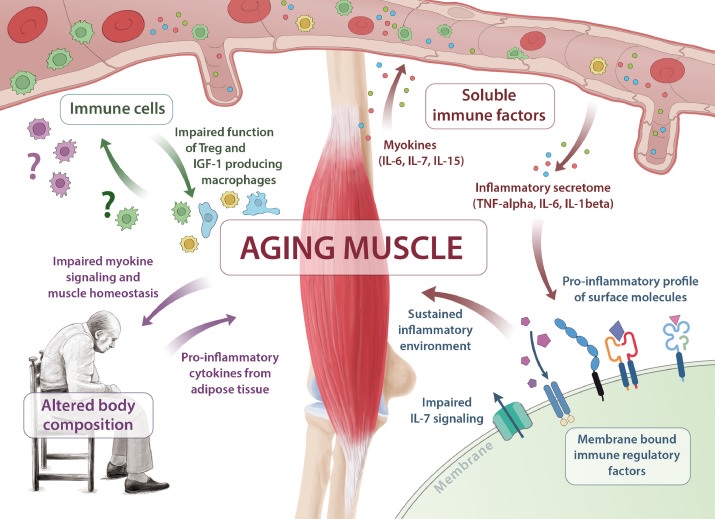 Skeletal muscle aging is essential in the pathogenesis of immune senescence and sarcopenia. Multiple pathways are affected, including insufficient myokine signaling (IL-6, IL-7, IL-15), shift of membrane-bound immune regulatory factors toward a pro-inflammatory profile, altered immune cell function, and altered body composition .
Skeletal muscle aging is essential in the pathogenesis of immune senescence and sarcopenia. Multiple pathways are affected, including insufficient myokine signaling (IL-6, IL-7, IL-15), shift of membrane-bound immune regulatory factors toward a pro-inflammatory profile, altered immune cell function, and altered body composition .
Sarcopenia and immunosenescence: a bidirectional link
Skeletal muscle exhibits immune regulatory properties and chronic low-grade inflammation can induce loss of muscle mass. The concept of skeletal muscle as a regulator of immune function is relatively new and adds a new layer of complexity to the muscle-immune system link.
Consequently, the muscle-immune system connection could be bidirectional : chronic low-grade inflammation induces muscle catabolism through pleiotropic mechanisms mediated by the inflammatory secretome. At the same time, skeletal muscle homeostasis is, in part, responsible for healthy immune function.
However, when dysregulated, insufficient myokine signaling, alteration of membrane-bound factors toward a pro-inflammatory profile, and impaired regenerative capacity of immune cells can lead to disruption of immune system function.
We propose that biological aging may alter the balance of muscle immune system homeostasis with skeletal muscle acting as a potential central link between sarcopenia and immune senescence. Healthy muscle function is gradually lost in an aging biological system due to physical inactivity, metabolic changes, and the accumulation of chronic low-grade inflammation.
In turn, impaired muscle function restricts skeletal muscle cell signaling necessary for immune regulation and maintenance, accumulating in a vicious cycle in which immune system and muscle dysfunction sustain each other.
Sarcopenic patients have an increased risk of infection, implying a clinical correlate of altered immune function.
The impact of sarcopenia on immune function with respect to autoimmune diseases and cancer is less clear. The incidence of cancer and autoimmune disorders increases as people age, while, on average, muscle mass decreases. Although suggestive, causality cannot be deduced. Longitudinal studies investigating the influence of sarcopenia on the incidence of cancer and autoimmune diseases are needed.
Conclusion Skeletal muscle regulates immune system functions through myokine signaling and the expression of immunomodulatory surface molecules. Immune cells, in turn, critically influence muscle mass and function. Therefore, skeletal muscle may act as a central integrator between sarcopenia and immune senescence. Given their individual and socioeconomic burden, innovative therapeutic approaches are urgently needed and, if targeted at skeletal muscle, could restore both skeletal muscle and immune system function in aging individuals. |















