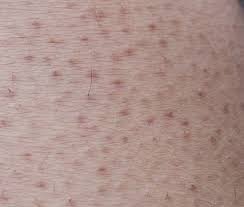
Global Preparedness Strategies for Rising Monkeypox Cases, Focus on Latin America
Countries like the US implement proactive strategies involving vaccine distribution and expanded testing to address rising monkeypox cases, prompting inquiries into the distribution of cases across Latin America and the need for regional response efforts.
March 2023

WHO Reports 61 Vaccines in Development for Drug-Resistant Bacterial Diseases
Despite promising advancements, the World Health Organization (WHO) emphasizes the ongoing need to enhance existing vaccine utilization as 61 potential vaccines for drug-resistant bacterial diseases are under development, albeit not immediately available.
March 2023
FDA Recommends Modification of Vaccines to Include Omicron BA 4/5 Component
The FDA advises the inclusion of an Omicron BA 4/5 spike protein component in COVID-19 vaccines, prompting efforts to develop modified vaccine formulations to enhance protection against emerging variants of concern.
March 2023