The US Food and Drug Administration (FDA) issued an emergency use authorization (EUA) for the JYNNEOS vaccine to allow healthcare providers to use the vaccine by intradermal injection to people 18 years of age and older at high risk of monkeypox infection. This type of administration will increase the total number of doses available for use by up to five times .
The EUA also allows the use of the vaccine in people under 18 years of age who are determined to be at high risk for monkeypox infection; In these individuals, JYNNEOS is administered by subcutaneous injection .
“In recent weeks, the monkeypox virus has continued to spread at a rate that has made it clear that our current vaccine supply will not meet current demand,” said FDA Commissioner Dr. Robert M. Califf . “The FDA quickly explored other scientifically appropriate options to facilitate access to the vaccine for all affected individuals. By increasing the number of doses available, more people who want to be vaccinated against monkeypox will now have the opportunity to do so.”
JYNNEOS, the modified Vaccinia Ankara (MVA) vaccine , was approved in 2019 for the prevention of smallpox and monkeypox disease in adults 18 years of age and older who were determined to be at high risk for smallpox. or monkeypox infection. JYNNEOS is administered under the skin (subcutaneously) in two doses, four weeks (28 days) apart.
For people over 18 years of age who are determined to be at high risk for monkeypox infection, the EUA now allows a fraction of the JYNNEOS dose to be administered between the layers of the skin (intradermally). Two doses of the vaccine administered four weeks (28 days) apart will still be needed.
There are no data available to indicate that one dose of JYNNEOS will provide long-lasting protection, which will be necessary to control the current monkeypox outbreak.
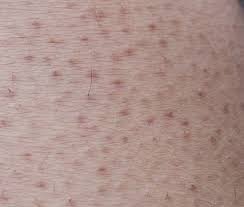
Data from a 2015 clinical study of the MVA vaccine evaluated a two-dose series administered intradermally compared to those administered subcutaneously. People who received the vaccine intradermally received a smaller volume (one-fifth) than people who received the vaccine subcutaneously . The results of this study demonstrated that intradermal administration produced a similar immune response to subcutaneous administration , meaning that individuals in both groups responded to vaccination similarly.
Intradermal administration caused more redness, firmness, itching, and swelling at the injection site, but less pain, and these side effects were manageable. The FDA has determined that the known and potential benefits of JYNNEOS outweigh the known and potential risks for the authorized uses.
To support the FDA’s authorization of two doses of JYNNEOS administered subcutaneously in people under 18 years of age, the FDA considered available data on the safety and immune response of JYNNEOS in adults, as well as historical data with the use of Live vaccinia vaccine against smallpox virus in pediatric populations.
JYNNEOS was tested in immunocompromised people and found to be safe and effective in trials conducted to support approval. It was initially developed specifically as an alternative for use in immunocompromised people in the event of a smallpox outbreak.