Tixagevimab-cilgavimab is a combination medicine to help prevent COVID-19 infection.
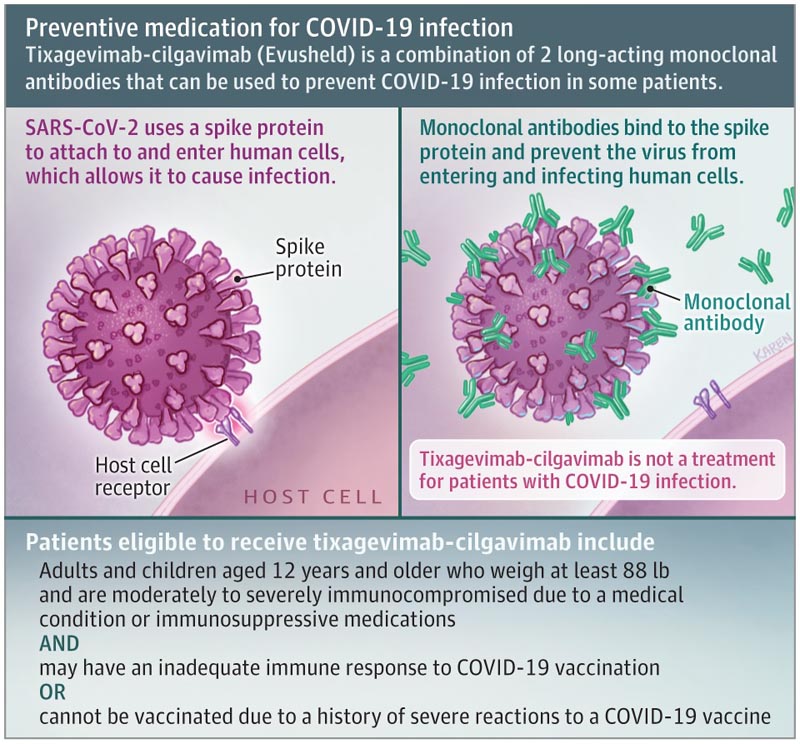
Tixagevimab-cilgavimab (Evusheld) consists of 2 long-acting monoclonal antibodies that bind to the spike protein of the virus that causes COVID-19 to prevent it from infecting human cells.
When can Tixagevimab-Cilgavimab be administered?
Tixagevimab-cilgavimab is not a treatment for COVID-19 and should not be given to patients who are already infected with COVID-19 or who had recent close contact with a person who has COVID-19 infection.
Administration of tixagevimab-cilgavimab should be deferred for at least 2 weeks after receiving the COVID-19 vaccine .
Who is eligible to receive Tixagevimab-Cilgavimab?
On December 8, 2021, the US Food and Drug Administration issued an Emergency Use Authorization (EUA) for tixagevimab-cilgavimab. Eligible patients include adults and children 12 years of age and older who weigh at least 40 kg (88 lb) and are moderately to severely immunocompromised due to a medical condition or immunosuppressive medications and may have an inadequate immune response to the COVID vaccine. -19 or cannot be vaccinated due to a history of severe reactions to a COVID-19 vaccine.
Where and how is Tixagevimab-Cilgavimab administered?
Tixagevimab-cilgavimab is administered as 2 separate consecutive intramuscular injections during a single session in a doctor’s office, infusion center, or other healthcare facility. Individuals are monitored for adverse site reactions for at least 1 hour after receiving tixagevimab-cilgavimab.
If continued protection against COVID-19 is needed, tixagevimab-cilgavimab is currently administered every 6 months . People who meet the eligibility criteria for tixagevimab-cilgavimab should contact their doctor to find out where to receive this treatment, which is provided to patients at no cost.
How effective is Tixagevimab-Cilgavimab?
Tixagevimab-cilgavimab has been shown to substantially reduce the risk of developing symptomatic COVID-19 infection for up to 6 months after administration. Research into the protection provided by tixagevimab-cilgavimab is ongoing, especially because mutations in the spike protein of emerging variants of the virus that causes COVID-19 could interfere with the effectiveness of tixagevimab-cilgavimab.
Possible side effects of Tixagevimab-Cilgavimab
Possible side effects of receiving an intramuscular medication include pain, swelling, bruising, and possible bleeding or skin infection at the injection site. Allergic reactions may occur during or after administration of tixagevimab-cilgavimab and may include symptoms such as difficulty breathing, chest pain, hives, wheezing, and swelling of the face, lips, mouth, and tongue. People who have had a severe allergic reaction to a COVID-19 vaccine are at increased risk of having an allergic reaction to tixagevimab-cilgavimab. Rare serious cardiac adverse events have been reported in patients with underlying cardiac risk factors receiving tixagevimab-cilgavimab.
What happens if a patient who received tixagevimab-cilgavimab develops symptoms of COVID-19?
People who develop symptoms of COVID-19 infection should get tested for the virus as soon as possible. Patients who received tixagevimab-cilgavimab and later tested positive for COVID-19 may be eligible for treatment, so they should contact their doctor to discuss the best therapeutic options available for COVID-19.
For more information: FDA, US Food and Drug Administration.