Summary Ensuring high vaccination and even booster vaccination coverage is critical to preventing severe coronavirus disease 2019 (COVID-19). Among the various COVID-19 vaccines currently in use, mRNA vaccines have demonstrated notable efficacy. However, systemic adverse events (AEs), such as post-vaccination fatigue, are common after mRNA vaccination and the basis for them is not known. Here, we found that higher baseline expression of genes related to T and NK cell exhaustion and suppression was positively correlated with the development of moderately severe fatigue after vaccination with Pfizer-BioNTech BNT162b2; Increased expression of genes associated with T and NK cell exhaustion and suppression that responded to vaccination was associated with higher levels of innate immune activation one day after vaccination. Furthermore, we found, in a mouse model, that altering the vaccination route from intramuscular (im) to subcutaneous (sc) could decrease the proinflammatory response and, consequently, the extent of systemic AEs; the humoral immune response to BNT162b2 vaccination was not compromised. Instead, it is possible that the sc pathway could enhance cytotoxic CD8 T cell responses to BNT162b2 vaccination. Therefore, our findings provide insight into the molecular basis of post-vaccination fatigue from mRNA vaccination and suggest an easily translatable solution to minimize systemic adverse events. |
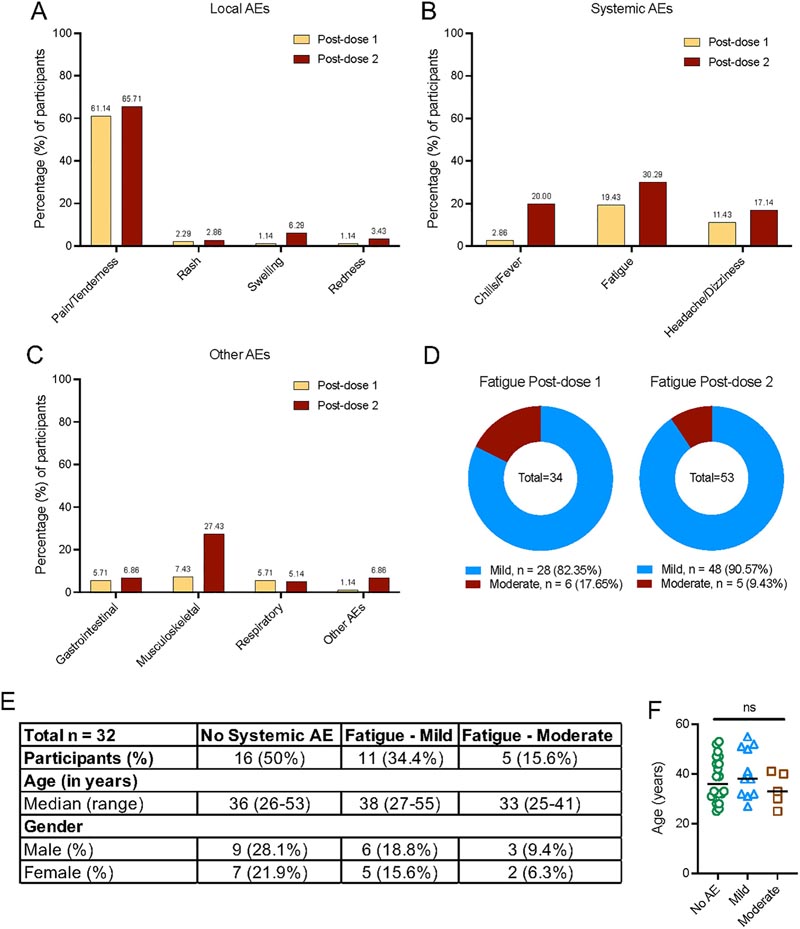
Local and systemic AEs reported after vaccination with Pfizer-BioNTech (BNT162b2) vaccine (n = 175). (A) Percentage of participants reporting local adverse events at the injection site after doses 1 and 2 of vaccination. (B) Percentage of participants reporting systemic AEs after doses 1 and 2 of vaccination. (C) Percentage of participants reporting AEs associated with respiratory, gastrointestinal, musculoskeletal, and other AEs reported not belonging to any category. (D) Percentage of participants reporting fatigue classified by severity (mild and moderately severe) after doses 1 and 2 of vaccination. (E) Demographic data of selected participants in the nested case-control study. (F) Age of participants classified according to fatigue severity. The data underlying this figure can be found in S1 Data. AE, adverse event.
Comments
Despite their strong efficacy against SARS-CoV-2, mRNA-based COVID-19 vaccines are associated with post-vaccination adverse effects such as fatigue; How can this be avoided?
In a new study published in the open access journal PLOS Biology , Ayesa Syenina of Duke-NUS Medical School in Singapore and colleagues report that a new analysis of blood samples from people vaccinated against COVID-19 identified distinct molecular characteristics linked to a higher likelihood of post-vaccination fatigue. Furthermore, experiments in mice suggest that changing the vaccine injection strategy could alleviate these adverse effects.
Post-vaccination adverse events can influence people’s willingness to get vaccinated or receive a booster dose, hampering efforts to reduce the spread and severity of COVID-19. However, the molecular underpinnings of adverse events following vaccination have been unclear.
To improve understanding, Syenina and colleagues analyzed blood samples from 175 healthcare workers who received BNT162b2, the Pfizer-BioNTech COVID-19 vaccine. Specifically, they used the blood samples to analyze a snapshot of each participant’s gene expression, or which genes are turned on or off.
This analysis revealed that people who experienced moderately severe fatigue after vaccination were more likely to have higher baseline expression of genes related to the activity of T cells and natural killer cells, two key cell types in the human immune system. .
The researchers also tested two different vaccination injection strategies in mice. Some mice received BNT162b2 by intramuscular injection, the current method used for human patients, in which the vaccine is injected into the muscles. Other mice received a subcutaneous injection , in which the vaccine is injected into the tissue just under the skin.
After vaccination, compared to mice that received intramuscular vaccination, mice that received subcutaneous vaccination showed immune system responses that are in line with a lower likelihood of adverse effects such as fatigue. However, subcutaneous injection did not appear to compromise the protective effects of vaccination.
Further research will be needed to build on these findings and explore their clinical significance. Still, they improve understanding of post-vaccination fatigue and offer a potential strategy to reduce its likelihood.
Co-author Eng Eong Ooi adds: “This study provides the first insight into the molecular basis of a side effect that many have experienced after mRNA vaccination. “We hope this finding will prompt further studies to fully understand the underlying mechanisms behind vaccine-associated side effects and collectively contribute to developing even more tolerable vaccines.”