Aim To review the outcomes of patients who underwent autologous oocyte thawing after planned oocyte cryopreservation. Design Retrospective cohort study. Adjustment Large fertility center affiliated with an urban university. Patient(s) All patients who underwent ≥1 autologous oocyte thawing before December 31, 2020. Intervention(s) None. The main measures) The primary outcome was the final live birth rate (FLBR) per patient, and only patients who had a live birth (LB) or who consumed all remaining inventory (cryopreserved oocytes and euploid/untested/no resulting results) embryos were included. . Secondary outcomes were laboratory results and LB rates per transfer. Results A total of 543 patients underwent 800 oocyte cryopreservations, 605 thawings, and 436 transfers. The median age at the first cryopreservation was 38.3 years . The median time between the first cryopreservation and thawing was 4.2 years . The median number of oocytes and metaphase II (M2) oocytes thawed per patient was 14 and 12, respectively. The overall survival of all thawed oocytes was 79%. Of all patients, 61% underwent ≥1 transfer. Among euploid (n = 262) and non-biopsy (n = 158) transfers, BL rates per transfer were 55% and 31%, respectively. The FLBR per patient was 39%. Age at cryopreservation and number of thawed M2 were predictive of LB; FLBR per patient was >50% for patients <38 years at cryopreservation or who thawed ≥20 M2s. A total of 173 patients (32%) have remaining inventory. Conclusion(s) Thawing of autologous oocytes resulted in a final live birth rate (FLBR) of 39% per patient , which is comparable to the results of age-matched in vitro fertilization. Studies with larger cohorts are necessary. |
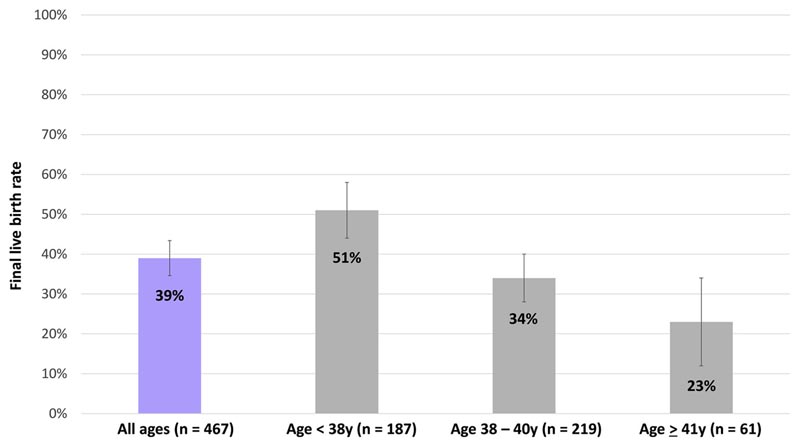
Final live birth rate per patient according to age at first cryopreservation; 95% confidence intervals are shown. Live births include 1 pregnancy with unknown outcome (ongoing at last contact). The median number of oocyte cryopreservation cycles for each group was 1. n = number of patients.
Comments
Not all women are ready to become mothers in their late teens, 20s, or even mid-30s, but a natural decline in fertility can make it more difficult to have a baby in your 40s and older.
Now, a new study shows that freezing eggs when they are younger is a very successful option for many women who are fertile in their younger years but want to delay pregnancy for a while.
Researchers looked at success rates of using a woman’s own frozen eggs for 15 years. They found that about 70% of women who had their eggs frozen when they were under 38 , and thawed at least 20 of those eggs, later had a baby.
Not only that, but freezing the eggs and then thawing them at a later date provided a higher pregnancy success rate than using fresh embryos with assisted reproductive technology.
"We’ve been very cautious and careful in publishing the data because we want it to be the right and accurate story. And we want women to be informed," said study author Dr. James Grifo, director of the division of reproductive health. endocrinology and infertility at NYU Langone Fertility Center in New York City. "Essentially, yes, you can conclude that if you freeze a batch of eggs at 30 and need IVF at 40, you have a better chance."
Most previous research on the question of the possibility of births from frozen eggs has been based on mathematical models, unlike this study, which was based on clinical experience.
The study was conducted at New York University Langone Fertility Center, where the first baby born through egg freezing will soon turn 17. Typically, younger women , and therefore with younger eggs, have higher pregnancy rates and lower miscarriage rates.
"We recognized that egg freezing was a way for a young woman to put her eggs ’on ice,’" Grifo said, noting that previous research included mouse models.
This study included 543 patients, with an average age of 38 years , who had their eggs extracted and frozen. That’s older than the optimal age for freezing eggs, which is before age 35. The patients underwent 800 egg freezing cycles, 605 egg thawing cycles, and 436 embryo transfers using the eggs between 2005 and 2020.
Overall, 39 percent of women ages 27 to 44 (most between ages 35 and 40) had at least one child from their frozen eggs when their eggs were frozen, the researchers found. That is comparable to the results of the same age in IVF (in vitro fertilization).
Across all the different age ranges of the study patients, those who thawed 20 mature eggs had a live birth rate of 58 percent, which the authors called "profound and unexpected," considering the women were already past their prime. reproductive.
Even 14 of the women who did not freeze their eggs until age 41 or 42 had children with frozen eggs. It didn’t seem to matter how long the eggs were stored, the researchers noted.
The study also found that genetic screening of embryos from frozen eggs before implantation also resulted in lower rates of miscarriage and a higher number of live births.
In total, 211 babies were born from egg freezing during the study, which was recently published online in the journal Fertility and Sterility .
That compares with less than 30 percent of getting pregnant when using fresh eggs or embryos for women in their 40s undergoing IVF and less than 20 percent of giving birth to live babies, according to statistics from the Centers for Disease Control and Prevention. Disease Prevention and Prevention collected data from nearly 500 U.S. centers.
Frozen eggs work as well as frozen embryos, Grifo said.
While the study looked at women with an average age of 38, the average age for egg freezing at the fertility clinic has now dropped over the past five years to 34. Grifo said she expects even better future results.
The center is seeing the number of women freezing eggs triple in 2022 compared to 2019. Dr. Timothy Hickman is medical director of CCRM in Houston and president of the Society for Assisted Reproductive Technology. He said: "I’m glad it’s published because we really needed data like this to see what the outcomes are for all these frozen eggs that we’ve been freezing for years and years."
Although women have been freezing eggs for many years, the data have been slow to emerge, Hickman added. "It’s difficult to collect because there is a long time between the time the eggs are frozen and the time they are actually thawed and used," she explained.
"Anyone who is interested in having a child at some point and isn’t ready to have one currently, then that’s usually the best type of patient that would approach this," Hickman said. "Now, it’s usually best to do it in your late 20s or early 30s, and it’s generally not recommended if you’re over 38. But here we’ve had patients over 38 or early 40s who have still used this and they have been able to have a child.
Egg retrieval is not an inexpensive process. It is similar to egg retrieval for IVF, but instead of producing embryos from the retrieved eggs, the eggs are frozen until the woman is ready and doctors can produce the embryos from selected sperm and the frozen eggs. of the woman.
Grifo estimated that it costs approximately $15,000 to go through the process and about $1,000 a year to store the frozen eggs and more again when embryos are created using the frozen eggs.
Hickman noted that he has seen the rates at which insurance companies cover IVF grow substantially over time.
"Fortunately, we’ve seen that here in Houston, a lot of corporations have been purchasing IVF coverage and often increasing coverage for their employees, so we’re very happy," Hickman said.
Although freezing eggs seems to provide good results for many, it is still not a guarantee of pregnancy. Even if someone appears to be fertile on tests performed at the time of recovery, the only true test of fertility is becoming pregnant and carrying a baby to term, Grifo noted.
|















