Although a decrease in protection against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has been observed in several studies after two doses of the BNT162b2 vaccine (Pfizer-BioNTech), the level of protection remains unchanged. be clear, as is the presence or degree of waning of natural immunity.
Several studies have shown that 6 or more months after infection, people still have substantial natural immunity against SARS-CoV-2. However, a recent study showed that messenger RNA (mRNA)-based vaccines confer a level of protection against hospitalization that is five times higher than that provided by prior infection.
The decreased humoral response of the immune system is well documented in vaccinated people and in those who have been infected with SARS-CoV-2. Additionally, studies of seasonal coronaviruses have shown a decrease in natural immunity and the possibility of reinfection. It is also unclear how natural immunity interacts with immunity conferred by vaccination.
Some laboratory studies have indicated that “hybrid immunity” (i.e., immunity conferred by the combination of prior infection and vaccination) offers greater broad-spectrum protection, elicits higher levels of neutralizing antibodies, and provides greater protection against infection than the immunity conferred by vaccination or infection alone. The durability of immunity resulting from SARS-CoV-2 infection and how this immunity compares to that conferred by vaccination are essential questions both at the level of an individual person and at the national level.
In this study, we estimated the incidence of confirmed SARS-CoV-2 infection in the following cohorts: previously infected, unvaccinated people ; previously infected people who had also received the BNT162b2 vaccine; and vaccinated people who had not previously been infected .
For each cohort, we quantified the association between time since infection or vaccination and the rate of confirmed infection. By comparing infection rates between these groups, we were able to assess the level of protection provided by hybrid immunity compared to that provided by natural immunity or immunity conferred by vaccination.
Background
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection provides natural immunity against reinfection. Recent studies have shown a decrease in immunity provided by the BNT162b2 vaccine. The time course of natural and hybrid immunity is unknown.
Methods
Using the Israeli Ministry of Health database, we extracted data from August and September 2021, when the B.1.617.2 (delta) variant was predominant, from all people who had previously been infected with SARS-CoV-2 or who had contracted coronavirus vaccine 2019. We used Poisson regression with adjustment for confounders to compare infection rates as a function of time since the last immunity-conferring event.
Results
The number of cases of SARS-CoV-2 infection per 100,000 person-days at risk (adjusted rate) increased with time since vaccination with BNT162b2 or since previous infection.
Among unvaccinated people who had recovered from infection, this rate increased from 10.5 among those who had been infected 4 to less than 6 months previously to 30.2 among those who had been infected 1 year or more previously.
Among people who had received a single dose of vaccine after a previous infection, the adjusted rate was low (3.7) among those who had been vaccinated less than 2 months previously, but increased to 11.6 among those who had been vaccinated. vaccinated at least 6 months before.
Among previously uninfected people who had received two doses of the vaccine , the adjusted rate rose to 21.
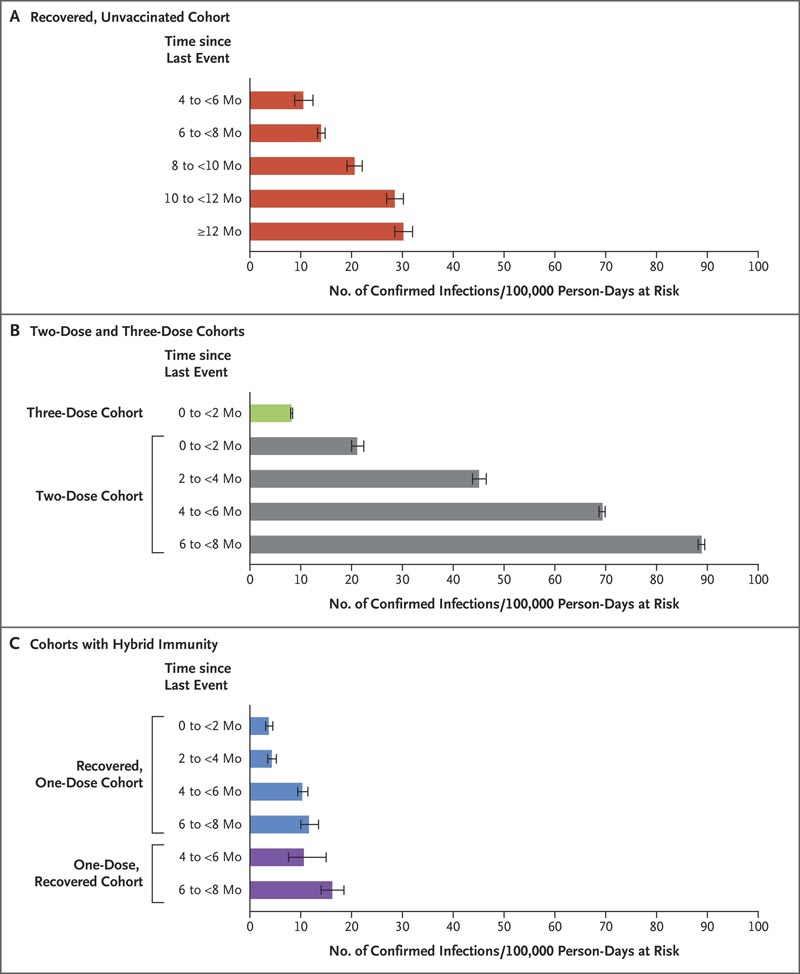
Estimated covariate-adjusted rates of confirmed infections per 100,000 person-days at risk. Data were obtained from Poisson regression analysis for the study period, stratified according to subcohorts. Confidence intervals are not adjusted for multiplicity. Error bars denote 95% confidence intervals.
Conclusions Among people who had previously been infected with SARS-CoV-2 (regardless of whether they had received any vaccine doses or whether they had received a dose before or after infection), protection against reinfection decreased as time increased since the last immunity - conferring event; However, this protection was greater than that conferred after the same time elapsed after receiving a second dose of vaccine among previously uninfected people. A single dose of vaccine after infection strengthened protection against reinfection. |
Discussion
Decreased immunity was evident in all cohorts. This pattern of waning immunity was evident across all age groups. Adjusted rates of confirmed infection among the recovered unvaccinated subcohorts were lower than those in the two-dose subcohorts when the time since the last immunity-conferring event was similar; however, protection in the two-dose cohort could be restored by administering a booster shot.
Understanding rates of immunity waning following immunity-conferring events is important for policymaking regarding the need and timing of additional vaccine doses. We found that protection against the delta variant decreased over time in both vaccinated and previously infected people and that an additional dose of vaccine restored protection.
Another article on the subject
Source : Gazit S et al. Short-term relative effectiveness of four doses versus three doses of the BNT162b2 vaccine in people aged 60 years and older in Israel : a retrospective, test-negative case-control study. BMJ 2022; 377:e071113. (https://doi.org/10.1136/bmj-2022-071113. opens in new tab)
After a fourth dose in an Israeli study, the vaccine’s effectiveness against infection decreased rapidly in those over 60 years of age, while prevention of severe disease was maintained for ≥10 weeks .
Expectations that a booster dose would provide long-lasting protection against SARS-CoV-2 infection have faded due to waning vaccine-induced immunity and the emergence of new SARS-CoV-2 variants.
In a retrospective case-control study of data from a large healthcare service, researchers in Israel have now assessed whether a fourth dose of the BNT162b2 (Pfizer-BioNTech) vaccine would bolster the short- and long-term protective effects of immunization among those eligible for COVID -19–naïve individuals.
Among 97,500 participants, 29% received a fourth vaccine dose. Because these recipients had more chronic diseases than those who received only three doses, the analysis was matched for confounding factors such as time of first test and month of receipt of the third dose and adjusted for comorbidities and immunosuppressive conditions.
The vaccine’s effectiveness against infection after receiving four or three doses peaked at 65% during the third week after vaccination, but quickly dropped to 22% at the end of 10 weeks of follow-up.
Severe COVID-19 was prevented with a relative effectiveness of 72% during follow-up.
In the real world, it is difficult to differentiate the decline in protection after sequential doses of the vaccine from the emergence of new SARS-CoV-2 variants. Additionally, relatively short intervals between doses are allowed in Israel, although longer intervals provide better immunity.
The study also does not answer whether a modified booster would have been more effective than additional doses of the original vaccine based on the Wuhan strain, a key question given the considerable changes that arise with the Omicron variant. To prepare for the next wave of COVID-19, these issues must be addressed.
Conclusions
A fourth dose of the BNT162b2 vaccine appears to have provided additional protection against SARS-CoV-2 infection and severe Covid-19 disease relative to three doses of the vaccine. However, the relative effectiveness of the fourth dose against infection appears to decline sooner than that of the third dose.