Highlights In this trial of IV vitamin C in adult patients with sepsis , those who received a vitamin C infusion had a higher risk of death or persistent organ dysfunction at 28 days than those who received placebo. |
Background
Studies that have evaluated the use of intravenous vitamin C in adults with sepsis who were receiving vasopressor therapy in the intensive care unit (ICU) have shown mixed results regarding the risk of death and organ dysfunction.
Methods
In this randomized, placebo-controlled trial , we assigned adults who had been in the ICU for no more than 24 hours, who had known or suspected infection as a primary diagnosis, and who were receiving a vasopressor to receive a vitamin C infusion (at a dose of 50 mg per kilogram of body weight) or placebo administered every 6 hours for up to 96 hours.
The primary outcome was a composite of death or persistent organ dysfunction (defined by use of vasopressors, invasive mechanical ventilation, or new renal replacement therapy) at day 28.
Results
A total of 872 patients were randomized (435 to the vitamin C group and 437 to the control group). The primary outcome occurred in 191 of 429 patients (44.5%) in the vitamin C group and in 167 of 434 patients (38.5%) in the control group (relative risk, 1.21; confidence interval [ 95% CI, 1.04 to 1.40; p=0.01).
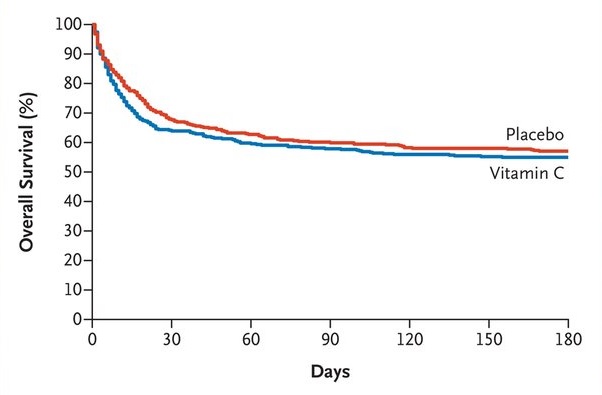
At 28 days , death had occurred in 152 of 429 patients (35.4%) in the vitamin C group and in 137 of 434 patients (31.6%) in the placebo group (hazard ratio, 1. 17; 95% CI, 0.98 to 1.40) and persistent organ dysfunction in 39 of 429 patients (9.1%) and 30 of 434 patients (6.9%), respectively (hazard ratio, 1. 30; 95% CI, 0.83 to 2.05).
Results were similar in the two groups with respect to organ dysfunction scores, biomarkers, 6-month survival, health-related quality of life, stage 3 acute kidney injury, and hypoglycemic episodes.
Conclusions
In adults with sepsis who received vasopressor therapy in the ICU, those who received intravenous vitamin C had a higher risk of death or persistent organ dysfunction at 28 days than those who received placebo.
(Funded by the Lotte and John Hecht Memorial Foundation; LOVIT ClinicalTrials.gov number, NCT03680274. opens in new tab.)















