Summary We suggest the use of MicroCLOTS (COVID-19 microvascular pulmonary obstructive thromboinflammatory syndrome) as a new name for severe pulmonary coronavirus disease 2019 (COVID-19). We hypothesize that, in predisposed individuals, alveolar viral damage is followed by an inflammatory reaction and microvascular pulmonary thrombosis. This progressive endothelial thromboinflammatory syndrome can also involve the microvascular bed of the brain and other vital organs, leading to multiple organ failure and death. Future steps in understanding the disease and identifying treatments may benefit from this definition and hypothetical sequence of events. |
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection represents a pandemic emergency of dramatic proportions.1
The clinical course of SARS-CoV-2 infection often meets the criteria for acute respiratory distress syndrome (ARDS), with progressive severity ultimately leading to rapid death.1-3
The pathophysiology of ARDS in severe cases of SARS-CoV-2 infection is attributed to a hyperimmune reaction of the host.3 From the first descriptions, it seemed that the progressive worsening of lung function in patients infected with SARS-CoV-2 was potentially driven by the host immune response.
SARS-CoV-2 enters target cells through the cell surface angiotensin-converting enzyme 2 (ACE2) receptor , which is expressed on the surface of lung epithelial cells and small intestinal enterocytes.
ACE2 receptors are also present on arterial and venous endothelial cells and arterial smooth muscle cells of multiple organs.5 Their replication causes direct cellular damage and the release of proinflammatory alarms from dying cells.6
In addition to this direct effect, viral particles can provoke host innate immune responses through different mechanisms, including activation of alveolar macrophages and the complement cascade through the lectin pathway.
Furthermore, locally formed immune complexes may play a role in further activating the complement system and increasing the inflammatory response as suggested by the recent finding of a large number of activated plasma cells in the bronchoalveolar lavage of a patient with severe coronavirus disease. 2019 (COVID -19) pneumonia.7
Activation of the complement cascade not only causes endothelial damage directly but also recruits leukocytes through the formation of C3a and C5a, responsible for a massive local release of proinflammatory cytokines such as interleukin (IL)-1, IL-6, IL-8 and interferon. -γ.8
| Within this massive host immune response, lymphocytes, resident macrophages, monocytes, and neutrophils exert their potent proinflammatory functions, causing additional severe injury to collateral tissue and massive damage to vascular endothelial and alveolar epithelial cells and microvascular thrombosis. 9.10 |
The functional implications of this peculiar pathogenesis of ARDS include a progressive worsening of ventilation/perfusion imbalances and a loss of hypoxic vasoconstriction reflexes, with a marked component of microvascular pulmonary thrombosis , as suggested by elevations of lactate dehydrogenase and D-dimer. eleven
| In the late stages of ARDS, the progression of endothelial damage with microvascular thrombosis can spread locally in the lung and potentially extend the systemic inflammatory reaction involving the microvascular bed of the kidneys, brain and other vital organs.12 |
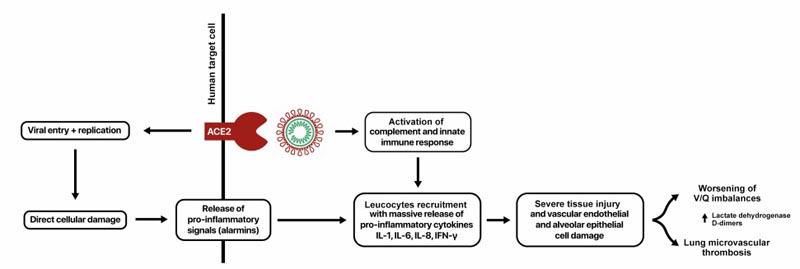 Figure 1. MicroCLOTS (COVID-19 microvascular pulmonary vasovascular obstructive thromboinflammatory syndrome) as a working hypothesis for atypical acute respiratory distress syndrome
Figure 1. MicroCLOTS (COVID-19 microvascular pulmonary vasovascular obstructive thromboinflammatory syndrome) as a working hypothesis for atypical acute respiratory distress syndrome
Since the beginning of the pandemic in Italy, our hospital underwent a profound reorganization to face the emergency, and we admitted to our institution more than 700 patients with severe SARS-CoV-2-induced pneumonia requiring oxygen therapy, including more of 100 patients admitted. to the intensive care unit.13
After our experience in managing patients and thanks to extensive multidisciplinary input, we now propose a mechanism of lung injury , mainly explained by dramatic alveolar endothelial damage leading to progressive pulmonary endothelial syndrome with microvascular thrombosis, and suggest MicroCLOTS ( microvascular pulmonary obstructive thromboinflammatory syndrome (COVID-19) as a working hypothesis of atypical ARDS (Figure 1).