Helicobacter pylori eradication for primary prevention of peptic ulcer bleeding in older patients prescribed aspirin in primary care (HEAT): a randomized, double-blind, placebo-controlled trial
Background
Peptic ulcers in patients receiving aspirin are associated with Helicobacter pylori infection. Our objective was to investigate whether H. pylori eradication would protect against aspirin-associated ulcer bleeding.
Methods
We conducted a randomized, double-blind, placebo-controlled trial (Helicobacter Eradication Aspirin Trial [HEAT]) in 1208 primary care settings in the UK, using routinely collected clinical data. Eligible patients were 60 years or older who were receiving aspirin at a daily dose of 325 mg or less (with four or more 28-day prescriptions in the past year) and had a positive C13 urea breath test for H. pylori in the selection. Patients receiving ulcerogenic or gastroprotective medication were excluded .
Participants were randomly assigned (1:1) to receive a combination of 500 mg oral clarithromycin , 400 mg metronidazole , and 30 mg lansoprazole (active eradication) or oral placebo (control), twice daily for 1 week.
Participants, their general practitioners and healthcare providers, and research nurses, trial team, adjudication committee, and analysis team were masked to group assignment throughout the trial. Follow-up was carried out by examining electronic data in primary and secondary care.
The primary outcome was time to hospitalization or death due to definite or probable peptic ulcer bleeding, and was analyzed using Cox proportional hazards methods in the intention-to-treat population.
This trial is registered in EudraCT, 2011-003425-96.
Results
Between September 14, 2012 and November 22, 2017, 30,166 patients underwent breath testing for H. pylori, 5,367 had a positive result, and 5,352 were randomly assigned to receive active eradication (n=2,677) or placebo (n=2675) and were followed for a median of 5.0 years (IQR 3.9-6.4).
Analysis of the primary outcome showed a significant deviation from proportional hazards assumptions (p=0·0068), requiring analysis in separate time periods. There was a significant reduction in the incidence of the primary outcome in the active eradication group in the first 2.5 years of follow-up compared with the control group (six episodes adjudicated as definite or probable peptic ulcer bleeding, rate 0.92 [95 % CI 0·41–2·04] per 1000 person-years vs 17 episodes, rate 2·61 [1·62–4·19] per 1000 person-years, hazard ratio [HR] 0.35 [CI 95 % 0·14–0·89]; p=0·028).
This advantage remained significant after adjusting for the competing risk of death (p=0.028), but was lost with longer follow-up (HR 1.31 [95% CI 0.55–3.11] in the post-treatment period). the first 2.5 years; p=0.54).
Reports of adverse events were actively solicited; Taste alteration was the most common event (787 patients).
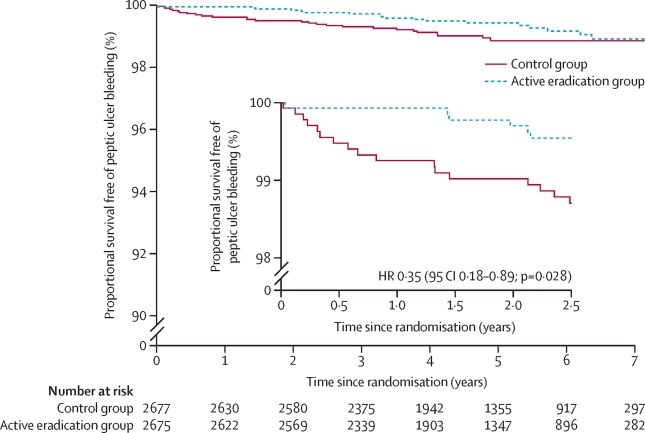
Kaplan-Meier curves for bleeding-free survival from peptic ulcer
Interpretation
H. pylori eradication protects against aspirin-associated peptic ulcer bleeding, but this may not be sustained in the long term.
Research in context
Evidence before this study
We searched MEDLINE, PubMed, the Cochrane Database of Systematic Reviews, and the Database of Abstracts and Reviews of Effects, without language or date restrictions, using the terms “aspirin,” “Helicobacter pylori,” and “peptic ulcer.” "before the study. began and repeated the search on October 20, 2022. Meta-analyses have shown that peptic ulcers and ulcer bleeding in patients receiving low-dose aspirin (≤325 mg daily) are strongly associated with Helicobacter pylori. This is compatible with the hypothesis that low-dose aspirin acts by enhancing bleeding from ulcers caused by H. pylori through its antihemostatic activity .
H. pylori eradication may prevent aspirin-induced acute endoscopic injury, but data on secondary prevention of recurrent ulcer bleeding are conflicting. To our knowledge, there have been no randomized trials on the effect of H. pylori eradication for the primary prevention of aspirin-associated ulcer bleeding nor have any studies been conducted in primary care.
Added value of this study
This trial demonstrated that H. pylori eradication can be reliably achieved in large populations of unselected older patients receiving aspirin at a dose of 325 mg or less in primary care. H. pylori eradication was associated with a significant reduction in the risk of hospitalization for ulcer bleeding, although this benefit was lost over time, a finding that had not been observed before.
Implications of all available evidence
The establishment of H. pylori eradication as an alternative or addition to antisecretory protection adds to the gastroprotective strategies available for the safe prescription of aspirin. The phenomenon of apparent loss of protection over time deserves further investigation. Our findings should prompt a reevaluation of strategies for safe aspirin prescribing and the balance of risks and benefits of its use in the prevention of cardiovascular disease and cancer. The trial also establishes a methodology that can be applied to the evaluation in primary care of other important clinical aspects.
Discussion
In this large trial of patients taking low-dose aspirin for several months in the previous year, we achieved high rates of H. pylori eradication and showed evidence of benefit, with a 65% reduction in hospitalizations due to peptic ulcer bleeding during the first 2·5 years in patients in the active eradication group compared to the control group. This finding was attributed to differences in gastric and duodenal ulcer bleeding.
However, this advantage appeared to be subsequently lost with longer follow-up.
There were no significant differences between groups in the incidence of uncomplicated ulcers or thrombotic cardiovascular events, and the incidence of dyspepsia was low. As expected, a considerable number of patients died, but competing risk analysis showed that our results for eradication treatment remained significant when adjusted for ongoing mortality rates. The large number of adverse events reported was expected, reflecting active data collection.
This trial expands the understanding of the effects of H. pylori eradication beyond the 12 months for which there was previous direct data and into the realm of primary prophylaxis. However, relatively few patients had bleeding ulcers, and only two of the 657 patients who died had peptic ulcer cited as the cause. A trend towards a lower mortality rate after eradication treatment was not expected.
The HEAT trial was a real-world study and prescribing changes, including discontinuation of aspirin or initiation of gastroprotective or ulcerogenic drugs, were permitted as clinically indicated or as recommended by consensus guidelines. However, differences between treatment groups remained significant in analyzes that adjusted for such drug use.
The number of patients in the control group with a negative repeat breath test at the end of the study was higher than expected. Home breath tests have been shown to be reliable, but can give false negative results , and it is also possible that exposure to clarithromycin and proton pump inhibitors contributed to this finding, due to eradication or incidental suppression of H. pylori.
The loss of protection against ulcers over time appears to be a real phenomenon that cannot be attributed to the increased use of gastroprotective drugs, which would have an opposite effect. Possible causes could be increased acid secretion or reduced release of protective prostaglandins after H. pylori eradication. Another possibility is that eradication of H. pylori will uncover a population of idiopathic ulcers with a high relapse rate.
Comments
A new study, led by experts at the University of Nottingham, found that the risk of stomach bleeding caused by long-term aspirin use can be reduced with a short course of antibiotics, which could improve the safety of aspirin when used to prevent heart attacks. strokes and possibly some types of cancer.
Results from the HEAT ( Helicobacter pylori Eradication Aspirin ) trial, which was led by Professor Chris Hawkey from the University of Nottingham’s Nottingham School of Medicine and Digestive Diseases Centre, and funded by the Technology Assessment program Health from the National Institute for Health and Care Research, are published in The Lancet .
Low-dose aspirin is a very useful preventive drug in people at high risk of stroke or heart attack. However, in rare cases, it can cause bleeding from internal ulcers. By thinning the blood, aspirin causes ulcers in the stomach to bleed. These ulcers can be caused by a particular type of bacteria, Helicobacter pylori.
The STAR (Simple Trials for Academic Research) team at the University of Nottingham investigated whether a short course of antibiotics to eliminate these bacteria would reduce the risk of bleeding in aspirin users.
The HEAT (Helicobacter pylori Eradication Aspirin) trial was a very large trial conducted in 1208 UK general practices. It was a real-life study that used clinical data routinely stored in GP and hospital records, rather than bringing patients back for trial follow-up visits.
The team wrote to 188,875 patients taking aspirin and 30,166 volunteered and participated in the study. Those who tested positive for H. pylori were randomized to receive antibiotics or placebos (dummy pills) and were followed for up to 7 years.
During the first two and a half years, those treated with antibiotics were less likely to be hospitalized due to ulcer bleeding than those given dummy tablets (6 versus 17). Protection occurred quickly: with those receiving placebos (dummy treatment), the first hospitalization for ulcer bleeding occurred after 6 days, compared with 525 days after antibiotic treatment.
Over a longer period of time, protection appeared to wane.
However, the overall rate of hospitalization for ulcer bleeding was lower than expected and this is in line with other evidence that ulcer disease is declining . The risks for people already taking aspirin are low. The risks are greatest when people first start taking aspirin, when H. pylori is probably worth looking for and treating.
Professor Chris Hawkey said: “Aspirin has many benefits in terms of reducing the risk of heart attacks and strokes in people at higher risk. There is also evidence that it is capable of slowing down certain types of cancer. The HEAT trial is the largest study of its kind in the UK and we are pleased that the findings have shown that ulcer bleeding can be significantly reduced after a one-week course of antibiotics. “The long-term implications of the results are encouraging in terms of safe prescribing.”
The results of the trial will be presented at the UEG ( United European Gastroenterology ) scientific meeting in Vienna, where it won a top prize of 10,000 euros for abstracts. The STAR team intends to use the award to sponsor a competition to support a collaboration with a research team that wants to leverage the STAR methodology.