Background
Dystrophic epidermolysis bullosa is a rare genetic blistering skin disease caused by mutations in COL7A1, which encodes collagen type VII (C7). Beremagene geperpavec (B-VEC) is an investigational topical gene therapy based on herpes simplex virus type 1 (HSV-1) designed to restore the C7 protein through the administration of COL7A1.
Methods
We conducted a phase 3 , double-blind, randomized intrapatient, placebo-controlled trial involving patients 6 months of age or older with genetically confirmed dystrophic epidermolysis bullosa.
For each patient, a pair of primary wounds was selected, matching the wounds based on size, region, and appearance. Wounds within each pair were randomly assigned in a 1:1 ratio to receive a weekly application of B-VEC or placebo for 26 weeks.
The primary endpoint was complete healing of treated compared to untreated wounds at 6 months.
Secondary endpoints included complete wound healing at 3 months and change from baseline to weeks 22, 24, and 26 in pain intensity during wound dressing changes, assessed with the use of a visual analog scale (scores range from 0 to 10, with higher scores indicating greater pain).
Results
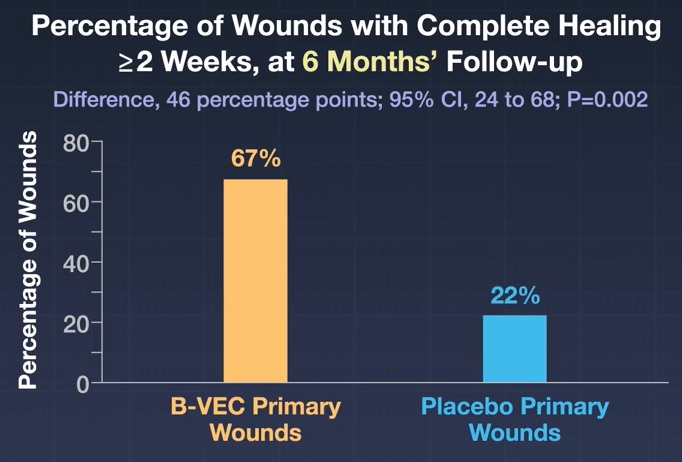
Pairs of primary wounds were exposed to B-VEC and placebo in 31 patients. At 6 months, complete wound healing occurred in 67% of wounds exposed to B-VEC compared with 22% of those exposed to placebo (difference, 46 percentage points; confidence interval [CI]. 95%, 24 to 68; P= 0.002).
Complete wound healing at 3 months occurred in 71% of wounds exposed to B-VEC compared with 20% of those exposed to placebo (difference, 51 percentage points; 95% CI, 29 to 73). ; P<0.001).
The mean change from baseline to week 22 in pain intensity during dressing changes was −0.88 with B-VEC and −0.71 with placebo (least squares-adjusted mean difference, −0.61; 95% CI, −1.10 to −0.13); Similar mean changes were observed at weeks 24 and 26. Adverse events with B-VEC and placebo included pruritus and chills.
Conclusions Complete wound healing at 3 and 6 months in patients with dystrophic epidermolysis bullosa was more likely with topical administration of B-VEC than with placebo. Pruritus and mild systemic side effects were observed in patients treated with B-VEC. Longer and larger trials are warranted to determine the durability and side effects of B-VEC for this disease. |
(Funded by Krystal Biotech; GEM-3 ClinicalTrials.gov number, NCT04491604















