Hydroxychloroquine ( HCQ ) was initially promoted as an oral therapy for the early treatment of coronavirus disease 2019 (COVID-19). Conventional meta-analyses cannot fully address the heterogeneity of different designs and results of randomized controlled trials (RCTs) evaluating the efficacy of HCQ in outpatients with mild COVID-19.
We performed a pooled analysis of individual participant data from RCTs evaluating the effect of HCQ on hospitalization and viral load reduction in outpatients with confirmed COVID-19.
We assessed the overall effect of treatment group using the log likelihood ratio (−2LL) test of a generalized linear mixed model to accommodate correlated longitudinal binary data. The analysis included data from 11 RCTs .
The virological effect result, evaluated in 1560 participants (N = 795 HCQ, N = 765 control), did not differ significantly between the two treatment groups (−2LL = 7.66; p = 0.18) when adjusting for cohort, duration of symptoms and comorbidities. The decrease in positive polymerase chain reaction tests from day 1 to 7 was 42.0 and 41.6 percentage points in the HCQ and control groups, respectively.
Among the 2037 participants evaluable for hospitalization (N = 1058 HCQ, N = 979 control), we found no significant difference in the rate of hospitalization between participants receiving HCQ and controls (odds ratio 0.995; 95% confidence interval 0.614– 1.610; −2LL = 0.0; p = 0.98) when adjusting for cohort, duration of symptoms, and comorbidities.
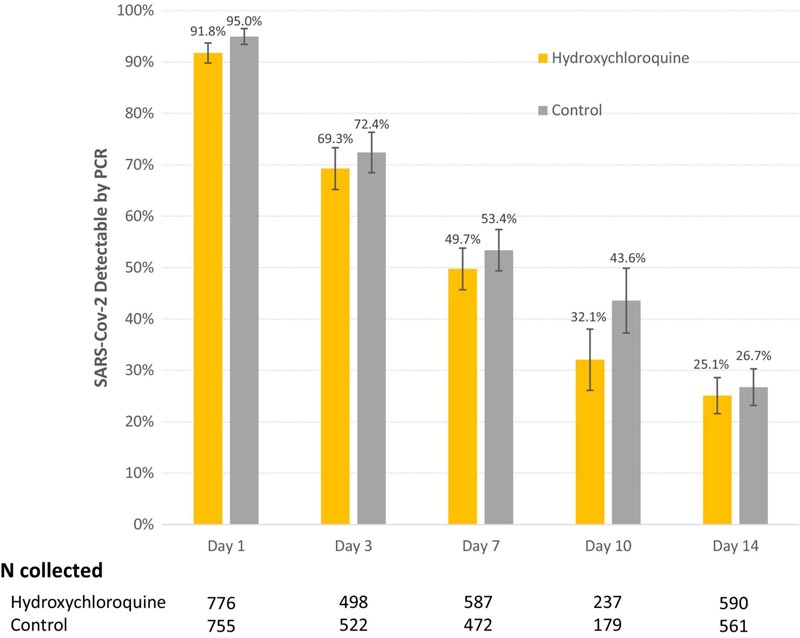
Figure: SARS-CoV-2 viral clearance with or without HCQ. Results based on nasal swab samples. The positive percentages are found among the samples collected. Uncollected samples may be true missing or not collected per protocol. Longitudinal mixed model analysis did not reveal a statistically significant effect of HCQ administration on viral load clearance over 14 days (−2LL = 7.66, p = 0.18). -2LL, log likelihood ratio test; HCQ, hydroxychloroquine; PCR, polymerase chain reaction; SARS-Cov-2, severe acute respiratory syndrome-coronavirus.
This meta-analysis of individual participant data from 11 HCQ trials evaluating severe acute respiratory syndrome-coronavirus 2 viral clearance and COVID-19 hospitalization did not show a clinical benefit of HCQ.
Our meta-analysis provides evidence to support discontinuation of HCQ use in outpatients with mild COVID-19 to reduce progression to severe disease.
Several randomized controlled trials have demonstrated little or no efficacy of hydroxychloroquine (HCQ) in treating mild coronavirus disease 2019 (COVID-19) in the outpatient setting; however, the investigated outcomes and results are heterogeneous and cannot be fully addressed by conventional meta-analyses of aggregated data.
In this meta-analysis of individual participant data, we found that HCQ does not improve viral clearance or reduce the risk of hospitalizations when administered to people with mild COVID-19.
Our meta-analysis provides evidence to support discontinuation of HCQ use in outpatients with mild COVID-19 to reduce progression to severe disease.
In summary , although HCQ is no longer a research priority, there is no convincing evidence on the effectiveness of HCQ in the treatment of outpatients with mild COVID-19. Our meta-analysis provides evidence to support discontinuation of HCQ use in outpatients with mild COVID-19 to reduce progression to severe disease.
















