Immune cell trafficking before hyperglycemia underlies subclinical diabetic cataratogenesis Summary Background This work clarifies the first cellular and molecular causes of cataratogenesis. The current paradigm presupposes elevated blood glucose levels as a prerequisite in diabetic cataratogenesis. Novel evidence in our diabetic cataract model challenges this notion and introduces immune cell migration to the lens and epithelial-mesenchymal transformation (EMT) of lens epithelial cells (LEC) as underlying causes. Methods The paucity of suitable animal models has hampered mechanistic studies of diabetic cataract, as most studies were traditionally carried out in acutely induced hyperglycemic animals. We introduced diabetic cataract in the Nile grass rat (NGR) that spontaneously develops type 2 diabetes (T2D) and showed its closeness to the human condition. Specialized stereoscopic microscopy with dual bright-field illumination revealed new dot-like hyperreflective microlesions in the inner cortical regions of the lens. To study the migration of immune cells to the lens, we developed a unique technique of in situ microscopy of the inner eyeball in combination with immunohistochemistry. Results Contrary to the existing paradigm, in approximately half of the animals, the newly introduced hyperreflective dot-like microlesions preceded hyperglycemia. Although the animals were normoglycemic, we found significant changes in their oral glucose tolerance test (OGTT), indicative of the prediabetic stage. The microlesions were accompanied by significant migration of immune cells from the ciliary bodies to the lens, as revealed by our novel in situ microscopy technique. Immune cells adhered to the lens surface, some passed through the lens capsule, and colocalized with apoptotic nuclei of lens epithelial cells (LECs). Extracellular degradations, amorphous material accumulations, and changes in E-cadherin expressions showed epithelial-mesenchymal transformation (EMT) in LEC. Subsequently, lens fiber disintegration and cataract progression extended to cortical, posterior, and anterior subcapsular cataracts. |
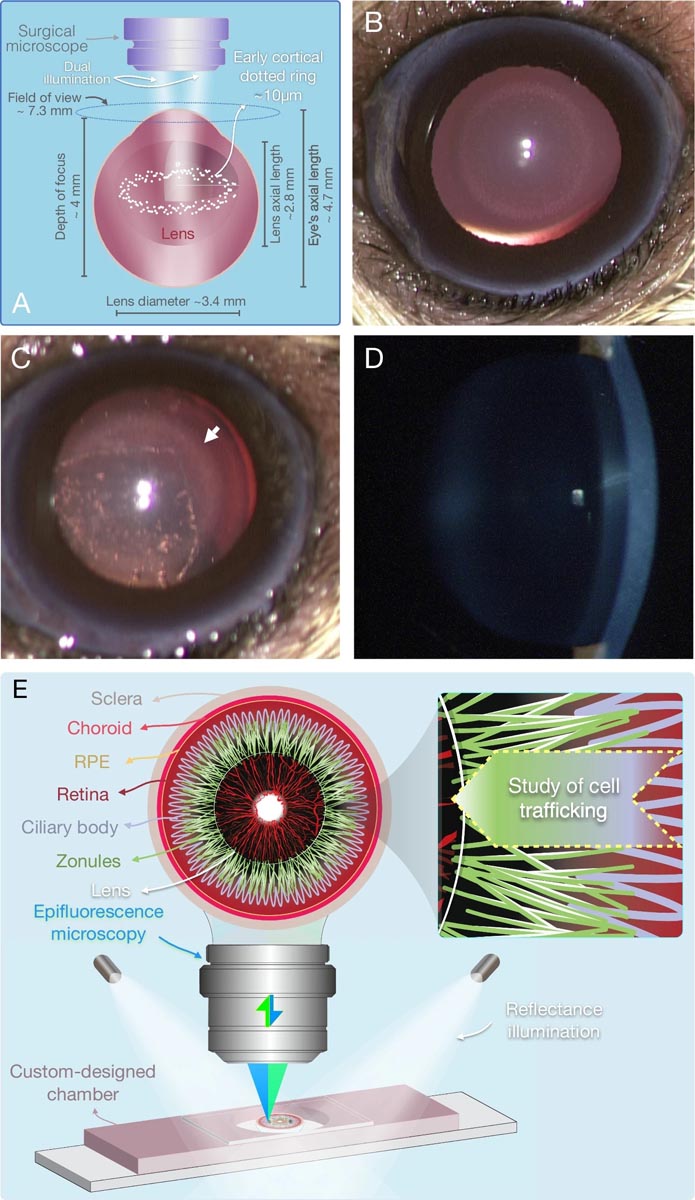
Figure : New enabling technologies in cataract images. A scheme of the dual illumination, necessary for the stereomicroscopic visualization of the new hyperreflective microlesions. B Bright field image of the first microlesions detectable in vivo in diabetic cataract at the punctate ring stage. C Stereomicroscopy of CEP along with the hyperreflective microlesions (arrow). D Lack of contrast for dot-like microlesions on slit-lamp biomicroscopy. E Overview of the novel anterior half-globe microscopy technique to image intact zonules in their original position. External illuminators in combination with epifluorescence microscopy provide a combined 3D image of ciliary bodies, zonules and migrating cells.
Conclusions
Our results establish a novel role for immune cells in LEC transformation and death. The fact that cataract formation precedes hyperglycemia challenges the prevailing paradigm that glucose initiates or is necessary to initiate pathogenesis.
Emerging evidence shows that the molecular and cellular complications of diabetes begin during the prediabetic state. These results have foreseeable ramifications for early diagnosis, prevention and the development of new treatment strategies in patients with diabetes.
Comments
The new findings from researchers at Brigham and Women’s Hospital, a founding member of the Mass General Brigham health care system, contradict previous notions about the role of sugar in the development of diabetic cataracts. Using an animal model that more closely recapitulates type 2 diabetes in humans, the research team found early signs of damage in the eye before the onset of type 2 diabetes, suggesting that diabetic complications may begin during the prediabetic state. . The results are published in the Journal of Biomedical Science .
A pitfall in the scientific process is not that a theory can be false, but that it is taken for granted when it seems logical and is not challenged with new experiments,” said Ali Hafezi-Moghadam, MD, PhD, director of Molecular Biomarkers Nano. -Imaging Laboratory (MBNI) in the Brigham Department of Radiology and associate professor at Harvard Medical School. "The evidence we have gathered here challenges the long-held theory of diabetic cataracts, and begs us to reexamine the current dogma that has been relied upon to explain cataracts associated with diabetes."
Cataracts, the clouding of the lens of the eye, are the leading cause of blindness worldwide and are a common complication of type 2 diabetes. The current hypothesis behind the development of diabetic cataracts is called the "sugar hypothesis" and suggests that High blood sugar, a hallmark of diabetes, precedes the development of cataracts. The working assumptions underlying the sugar hypothesis describe that higher levels of glucose in the lens of people with diabetes are converted into a sugar alcohol molecule called sorbitol, which induces structural changes in the lens of the eye that precede to the development of cataracts. While unproven, researchers rarely investigate this theory further due to the treatable nature of cataracts.
Hafezi-Moghadam and her colleagues studied the Nile grass rat, a model they originally reported develops type 2 diabetes spontaneously when kept in captivity and closely resembles the condition in humans. Like humans with type 2 diabetes, these animals first develop insulin resistance and high blood insulin levels before their blood glucose level rises above normal. Using a specialized technique called bright-field dual-illumination stereoscopic microscopy, the researchers observed the development of dot-shaped microlesions , which predisposed to cataract formation, in the inner cortical regions of the lens. Unexpectedly, they noticed that in almost half of the animals tested, these microlesions appeared before the animals developed hyperglycemia, or high blood sugar, suggesting that it was not elevated blood glucose levels that led to the development of cataracts.
Instead, the researchers identified that immune cells were migrating from specialized structures in the eye called ciliary bodies toward the lens. In these areas, where immune cells passed through the lens capsule, they found that the epithelial cells that normally cover the inner surface of the lens capsule changed identity and behaved differently. These changes, also known as epithelial-mesenchymal transformation (EMT), were followed by apparently disorganized cell growth, cell death, and cell migrations toward the lens body. In some regions, the newly transformed cells simply abandoned their original positions and made their way toward the lens. Such cellular changes, no matter how small in dimensions, significantly compromise the function of the lens.
While it is still too early to say what exactly causes immune cells and epithelial cells to behave the way they do, the researchers conclude that their study urges further investigation of prevailing theories. It may also bring the medical community closer to understanding the cellular mechanisms underlying the origins of diabetic complications during the prediabetic stage of the disease. And once we understand the pathogenesis, Hafezi-Moghadam predicts, we can begin to look at how to prevent people with diabetes from developing cataracts and potentially other complications elsewhere in the body.
“Although cataracts are easily removed with surgery today, this procedure carries the risk of complications and is costly, both for individuals and for our healthcare system,” Hafezi-Moghadam said. “With more than 500 million people worldwide and 37 million Americans having diabetes, the vast majority of whom have type 2, there is an incentive to try to find non-surgical ways to prevent, delay or even reverse this complication. . Perhaps one day it will be possible to avoid performing these surgeries altogether. And that requires us to go back to the basics of the cellular processes underlying cataract development.”
Funding : This work was supported by NIH Impact Award (DK108238-01, AHM) and JDRF)