Background
Respiratory syncytial virus (RSV) is an important cause of acute respiratory infection, lower respiratory tract disease, clinical complications, and death in older adults. There is currently no licensed vaccine against RSV infection.
Methods
In an ongoing, international, placebo-controlled, Phase 3 trial, we randomly assigned, in a 1:1 ratio, adults 60 years of age or older to receive a single dose of AS01 E-Protein-Based Vaccine Candidate. Respiratory syncytial virus (RSV) prefusion F with adjuvant (RSVPreF3 OA) or placebo before the RSV season.
The primary objective was to demonstrate the vaccine efficacy of one dose of RSVPreF3 OA vaccine against RSV-related lower respiratory tract disease, confirmed by reverse transcriptase polymerase chain reaction (RT-PCR), over one season. of VRS.
The criterion for meeting the primary objective was a lower limit of the confidence interval around the efficacy estimate of more than 20%. Efficacy against RSV-related severe lower respiratory tract disease and RSV-related acute respiratory infection was evaluated, and analyzes were performed according to RSV subtype (A and B). Safety was evaluated.
Results
A total of 24,966 participants received one dose of the RSVPreF3 OA vaccine (12,467 participants) or placebo (12,499). During a median follow-up of 6.7 months , vaccine efficacy against RSV-related lower respiratory tract disease confirmed by RT-PCR was 82.6% (confidence interval [CI] 96.95 %, 57.9 to 94.1), with 7 cases (1.0 per 1000 participant-years) in the vaccine group and 40 cases (5.8 per 1000 participant-years) in the placebo group.
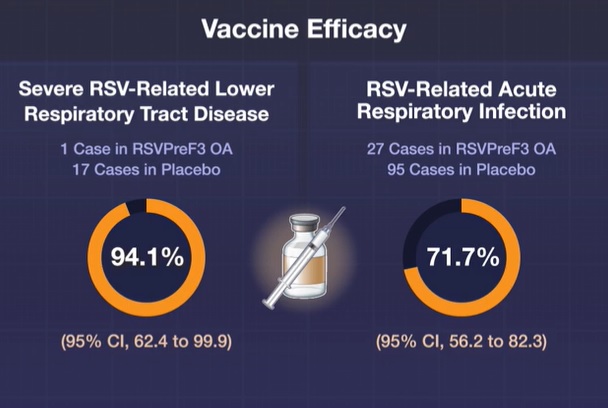
Vaccine efficacy was 94.1% (95% CI, 62.4 to 99.9) against RSV-related severe lower respiratory tract disease (assessed on the basis of clinical signs or by investigator) and 71.7% (95% CI, 56.2 to 82.3) versus RSV. -related acute respiratory infection.
Vaccine efficacy was similar against RSV subtypes A and B (for RSV-related lower respiratory tract disease: 84.6% and 80.9%, respectively; for RSV-related acute respiratory infection: 71. 9% and 70.6%, respectively).
High vaccine efficacy was observed in various age groups and in participants with coexisting conditions. The RSVPreF3 OA vaccine was more reactogenic than placebo, but most adverse events for which reports were requested were transient, with mild to moderate severity. The incidence of serious adverse events and possible immune-mediated diseases was similar in the two groups.
Conclusions A single dose of RSVPreF3 OA vaccine had an acceptable safety profile and prevented RSV-related acute respiratory infection and lower respiratory tract disease and RSV-related severe lower respiratory tract disease in adults aged 60 years. or more, regardless of RSV subtype and the presence of underlying coexisting conditions. |
(Funded by GlaxoSmithKline Biologicals; number AReSVi-006 ClinicalTrials.gov, NCT04886596. opens in new tab.)
















