Highlights
|
Heart failure is a global epidemic with increasing incidence, especially in the elderly. It is a syndrome derived from structural or functional cardiac anomalies, which lead to systolic and/or diastolic ventricular dysfunction. Advanced glycation end products ( AGEs) are formed after non-enzymatic reactions between proteins and sugar residues that accumulate in the human body. Its formation occurs through processes such as cooking and smoking cigarettes. AGEs accumulate throughout the body in the plasma and in the vessels, skin and heart tissue. The degree of non-enzymatic glycation depends on the glucose concentration and the exposure time. AGEs continually accumulate in vessel wall proteins and also in other tissues with aging and increase among people with diabetes , leading to complications such as diabetic microangiopathy . These end products have also been associated with cardiovascular disease and increased risk of mortality in the general population.
An association between AGEs and heart failure after acute decompensation has recently been reported. AGEs may contribute to heart failure through the induction of cardiac and vascular dysfunction. Primarily, glycation of extracellular matrix proteins within the vasculature and subsequent irreversible cross-linking of those proteins can lead to increased arterial stiffness and alterations in cardiac perfusion.
Objectives / Hypotheses
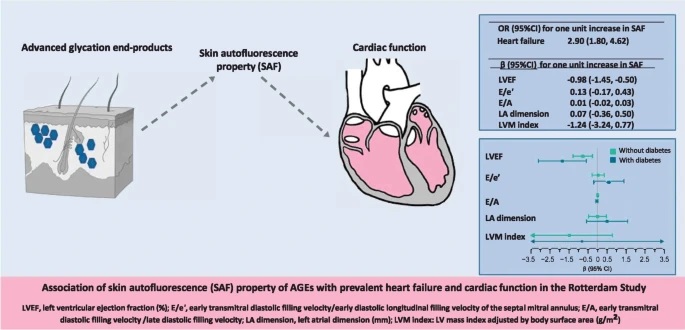
The objective of this work was to evaluate the association of advanced glycation end products (AGEs), measured by skin autofluorescence (SAF), with prevalent heart failure and with systolic and diastolic cardiac function, in a large cohort study. based on population.
Methods
We assessed the cross-sectional association between APS and prevalent heart failure among 2426 participants of the population-based Rotterdam Study using logistic regression.
Next, among people without heart failure (N = 2362), we examined the link between SAF (on a continuous scale) and echocardiographic parameters of left ventricular (LV) systolic and diastolic function using linear regressions. Analyzes were adjusted for traditional cardiovascular risk factors.
Results
Higher SAF levels were associated with higher odds of prevalent heart failure (multivariable adjusted OR 2.90 [95% CI: 1.80, 4.62] for one unit higher SAF value). Among individuals without heart failure, a one-unit increase in SAF was associated with a 0.98% lower LV ejection fraction (mean difference [β] -0.98% [95% CI: -1, 45%, -0.50%]).
The association was strongest among participants with diabetes (β -1.84% [95% CI: -3.10%, -0.58%] and β -0.78% [95% CI: -1 .29%, -0.27%] among participants with and without diabetes, respectively). Associations of SAF with parameters of diastolic function were not evident, except in men with diabetes.

Association of SAF with LV systolic and diastolic function parameters by type 2 diabetes status. Mean difference (β) (95% CI) for one unit increase in SAF was calculated using linear regressions adjusted for age, sex , coronary heart disease, hypertension, smoking, waist circumference, monthly income and physical activity. The number of participants without diabetes was 2069 and with diabetes was 357 in the original data. The arrows in the figure indicate that the CI interval exceeds the width of the x-axis
Conclusions
Higher SAF levels were associated with prevalent heart failure regardless of diabetes status. Among individuals without heart failure , higher SAF levels were associated with poorer LV systolic and diastolic function, although the associations were most prominent for systolic function.
Analyzes stratified by sex suggested an inverse association between AGEs and diastolic function parameters that was more prominent in men with diabetes. Further prospective investigation into the mechanisms linking AGEs to heart failure is warranted.
Discussion
In this population-based study, higher SAF levels were associated with prevalent heart failure. Among participants without clinical heart failure and regardless of diabetes status, higher SAF levels were accompanied by worse LV systolic function, while the association with diastolic function was less evident. Analyzes stratified by sex suggested an association between APS and worse diastolic function that was more prominent in men with diabetes.
AGEs are proposed targets in the treatment of heart failure. However, its role in the pathophysiology of cardiac function and heart failure has been studied primarily in people with chronic heart failure, diabetes, or coronary heart disease. In our study, higher SAF levels were associated with heart failure regardless of diabetes or CHD status. Furthermore, among people without heart failure, higher SAF levels were associated with worse LV systolic function. AGEs may negatively affect LV systolic function by accelerating atherosclerosis. They induce thrombosis and increase vasoconstriction.
Furthermore, by cross-linking with LDL, AGEs reduce its elimination and make these particles more atherogenic. They can also alter myocardial contraction by reducing intracellular calcium, leading to decreased myocardial contractility. On the other hand, excessive cross-linking as a result of AGE accumulation can reduce matrix flexibility and increase cellular stiffness in the heart. Impaired relaxation due to the same reduction in intracellular calcium and prolonged repolarization can also add to this. Thus, AGE accumulation is also suspected to result in diastolic dysfunction.
Final message AGE accumulation was independently associated with prevalent heart failure. Among individuals without heart failure, AGEs were associated with cardiac function, particularly systolic function. This association was present in participants with and without diabetes and was more prominent in those with diabetes. |
















