Community-acquired pneumonia remains a major public health problem. Worldwide, there were 489 million lower respiratory tract infections in 2019. In the United States, more than 1.5 million adults are hospitalized annually for community-acquired pneumonia. In 2019, pneumonia was the ninth leading cause of death in the United States and the leading cause of death from infection (approximately 50,000 deaths). In high-income countries, the monthly death rate among hospitalized patients with community-acquired pneumonia is approximately 10 to 12%. 4,5 Among patients receiving some type of mechanical ventilation, mortality can reach 30%.
Pneumonia can lead to intense pulmonary and systemic inflammation, resulting in impaired gas exchange, sepsis and organ failure, and an increased risk of death. Glucocorticoids have powerful anti-inflammatory and immunomodulatory activities that mitigate the consequences of pneumonia.
Seven randomized controlled trials showed that glucocorticoids had positive effects in patients with community-acquired pneumonia of varying severity; However, with the exception of one trial, none of these trials showed a difference between groups with respect to mortality. A meta-analysis of six of these trials suggested that glucocorticoids reduced time to clinical stabilization and length of hospital stay without improving survival. Another meta-analysis that included open-label trials or those judged to be at risk of bias suggested that glucocorticoids reduced mortality among patients with severe community-acquired pneumonia, with moderate quality of evidence.
We conducted the Community-Acquired Pneumonia: Evaluation of Corticosteroids (CAPE COD) trial to evaluate whether early treatment with hydrocortisone reduced 28-day mortality among patients admitted to an intensive care unit (ICU) for severe community-acquired pneumonia. .
Background
It is unclear whether the anti-inflammatory and immunomodulatory effects of glucocorticoids can decrease mortality among patients with severe community-acquired pneumonia.
Methods
In this phase 3, multicenter, double-blind, randomized, controlled trial, we assigned adults who had been admitted to the intensive care unit (ICU) for severe community-acquired pneumonia to receive intravenous hydrocortisone (200 mg daily for 4 u 8 days as determined by clinical improvement, followed by tapering over a total of 8 or 14 days) or receive placebo. All patients received standard therapy, including antibiotics and supportive care. The primary outcome was death at 28 days.
Results
A total of 800 patients had been randomized when the trial was stopped after the second planned interim analysis. Data from 795 patients were analyzed.
By day 28, death had occurred in 25 of 400 patients (6.2%; 95% confidence interval [CI], 3.9 to 8.6) in the hydrocortisone group and in 47 of 395 patients ( 11.9%; 95% CI, 8.7 to 15.1) in the placebo group (absolute difference, −5.6 percentage points; 95% CI, −9.6 to −1.7; P = 0.006).
Among patients not on mechanical ventilation at baseline, endotracheal intubation was performed in 40 of 222 (18.0%) in the hydrocortisone group and 65 of 220 (29.5%) in the placebo group. (hazard ratio, 0.59; 95% CI, 0.40 to 0.86).
Among patients who were not receiving vasopressors at baseline, vasopressors were initiated on day 28 in 55 of 359 (15.3%) in the hydrocortisone group and in 86 of 344 (25.0%) in the placebo group. (hazard ratio, 0.59; 95% CI, 0.43 to 0.82).
The frequencies of hospital-acquired infections and gastrointestinal bleeding were similar in the two groups; Patients in the hydrocortisone group received higher daily doses of insulin during the first week of treatment.
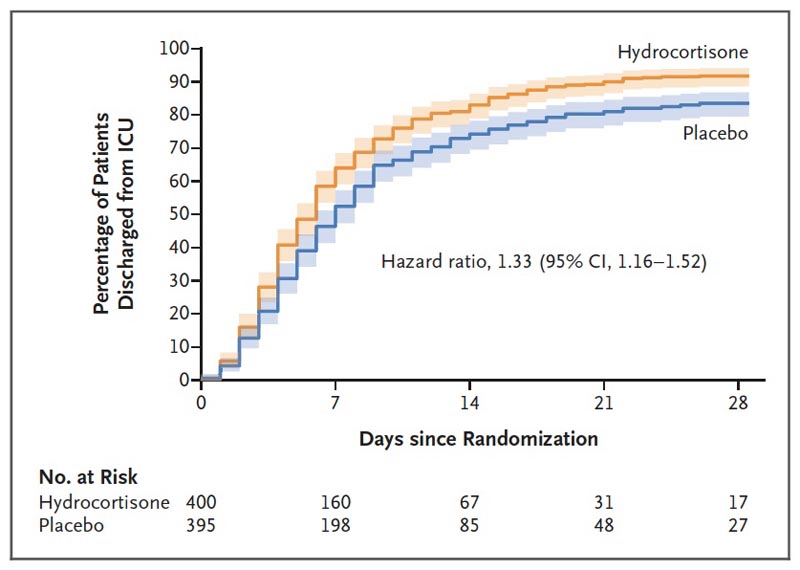
Figure . ICU discharge on day 28. The cumulative percentage of patients who were discharged from the ICU on day 28 (a secondary outcome of the trial) is shown. ICU length of stay was compared within the framework of a competing risk model, considering death as a competing event. For secondary outcomes, the widths of the confidence intervals have not been adjusted for multiplicity and cannot be used in place of hypothesis testing.
Conclusions Among patients with severe community-acquired pneumonia treated in the ICU, those receiving hydrocortisone had a lower risk of death at day 28 than those receiving placebo. |
Discussion
In this large multicenter trial, early hydrocortisone therapy reduced the rate of death at day 28 among patients who had been admitted to the ICU for severe community-acquired pneumonia. The results appeared to be consistent across important subgroups. Our data do not indicate any particular safety concerns, including the absence of differences between groups in the occurrence of hospital-acquired infections.
Few large multicenter trials have evaluated glucocorticoids in patients with severe community-acquired pneumonia who have been admitted to the ICU. In a trial involving 120 critically ill patients with community-acquired pneumonia and a C-reactive protein level of more than 15 mg per deciliter, treatment with methylprednisolone reduced a composite outcome of treatment failure but did not alter in-hospital mortality. . Results from a recently published trial showed no benefit from methylprednisolone in 584 patients hospitalized in the ICU for community-acquired pneumonia, with a day 60 mortality of 16% compared with 18% in the placebo group.
Several factors may explain these discrepancies.
- First, the pharmacodynamic properties of glucocorticoids may differ, including the balance between the effects of mineralocorticoids and glucocorticoids. In a previous small trial that suggested a decrease in mortality, patients also received hydrocortisone.
- Second, we excluded patients with septic shock at baseline because pathophysiological processes and the role of glucocorticoids may differ.
- Third, the median time between ICU admission and the first administration of hydrocortisone or placebo in our trial (<15 hours) may have promoted an early effect.
- Fourth, our trial population included a higher proportion of women (30.6%) than another trial in which glucocorticoid treatment did not alter mortality. and possible differences in the response to glucocorticoids according to sex have been suggested.
Hydrocortisone was not associated with an increase in hospital-acquired infections or gastrointestinal bleeding.
However, patients in the hydrocortisone group received higher doses of insulin during the first 7 days of treatment. An increased incidence of hyperglycemia has been reported in trials and meta-analyses , which is consistent with the pharmacodynamic effects of glucocorticoids. These increases are usually transitory, which we did not verify in the test.
Our trial has several limitations .
- First, the observed mortality of 11.9% in the control group was lower than expected (27%), which may indicate lower than expected disease severity. However, enrollment of a high-risk population is suggested by the percentage of patients who underwent mechanical ventilation, the distribution of Pneumonia Severity Index score, and the Pa o 2 : F o 2 ratio at baseline. of the study. However, we excluded patients with septic shock at the time of enrollment.
- Second, no standardized microbiological investigation was ordered and no pathogen was isolated in 357 of 795 patients (44.9%). Even in studies with extensive microbiological evaluations, no pathogen is detected in up to 62% of patients with community-acquired pneumonia.
- Third, we included a small percentage of immunocompromised patients, and the results should be applied with caution in this population.
- Fourth, we did not evaluate the reversibility of glucocorticoid-induced hyperglycemia. Furthermore, we did not specifically evaluate the possible neuropsychological and neuromuscular side effects of glucocorticoids.
- Fifth, the administration of hydrocortisone by continuous infusion and with decreasing doses compared to other potential regimens is not supported by a high level of evidence.
We found that early treatment with hydrocortisone reduced 28-day mortality among patients who had been admitted to the ICU with severe community-acquired pneumonia.
(Funded by the French Ministry of Health; CAPE COD ClinicalTrials.gov number, NCT02517489. opens in new tab.)















