Increased detection of small peripheral non-small cell lung cancer (NSCLC) has renewed interest in sublobar resection instead of lobectomy.
We conducted a multicenter, noninferiority , phase 3 trial in which patients with NSCLC clinically staged as T1aN0 (tumor size, ≤2 cm) were randomly assigned to undergo sublobar resection or lobar resection after intraoperative confirmation of node-negative disease.
The primary endpoint was disease-free survival, defined as the time between randomization and disease recurrence or death from any cause.
Secondary endpoints were overall survival, locoregional and systemic recurrence, and pulmonary functions.
From June 2007 to March 2017, a total of 697 patients were assigned to undergo sublobar resection (340 patients) or lobar resection (357 patients).
After a median follow-up of 7 years, sublobar resection was noninferior to lobar resection for disease-free survival (hazard ratio for disease recurrence or death, 1.01; 90% confidence interval [CI]. %, 0.83 to 1.24). Furthermore, overall survival after sublobar resection was similar to that after lobar resection (hazard ratio for death, 0.95; 95% CI, 0.72 to 1.26).
The 5-year disease-free survival was 63.6% (95% CI, 57.9 to 68.8) after sublobar resection and 64.1% (95% CI, 58.5 to 69). ,0) after lobar resection. The 5-year overall survival was 80.3% (95% CI, 75.5 to 84.3) after sublobar resection and 78.9% (95% CI, 74.1 to 82.3) after sublobar resection. 9) after lobar resection.
No substantial differences were observed between the two groups in the incidence of locoregional or distant recurrence. At 6 months postoperatively, a between-group difference of 2 percentage points was measured in the mean percentage of predicted forced expiratory volume in 1 second, favoring the sublobar resection group.
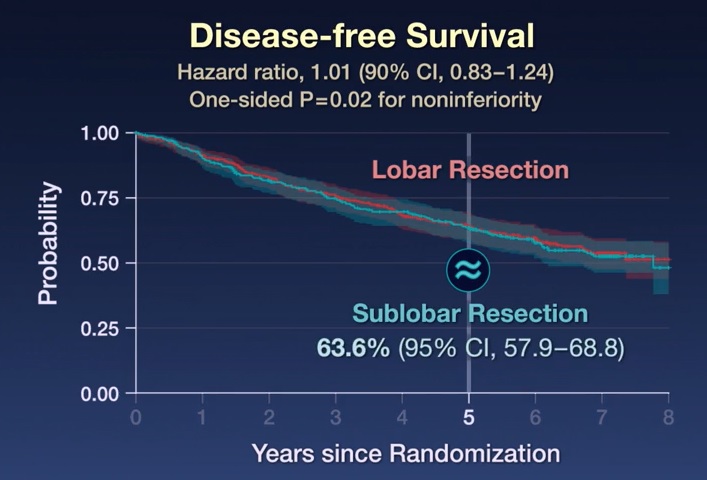
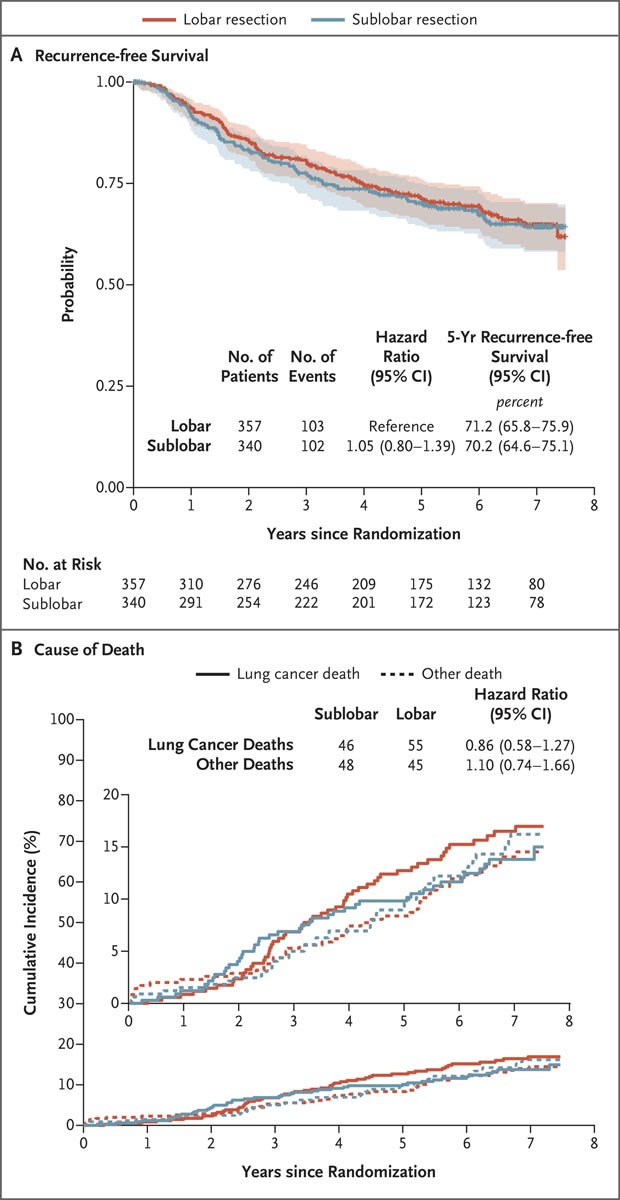
Figure: Recurrence-free survival and cause of death
Panel A shows recurrence-free survival in the intention-to-treat population. Recurrence-free survival was defined as the time between randomization and the occurrence of locoregional or distant recurrence; all other events, including death from any cause, were censored when these events occurred. Shaded areas indicate 95% confidence intervals. Panel B shows the cumulative incidence functions for lung cancer-related death compared to non-lung cancer-related death; Four patients with an unknown cause of death (three in the lobar resection group and one in the sublobar resection group) were excluded from the analysis. In both panels, the widths of the confidence intervals have not been adjusted for multiplicity and cannot be used in place of hypothesis testing.
In patients with peripheral non-small cell lung cancer (NSCLC) with a tumor size of 2 cm or less and pathologically confirmed node-negative disease in the hilar and mediastinal lymph nodes, sublobar resection was noninferior to lobectomy with respect to disease-free survival.
Overall survival was similar with both procedures.
In this large randomized trial, we found that in patients with NSCLC in peripheral clinical stage T1aN0 (≤2 cm), sublobar resection was noninferior to lobectomy with respect to disease-free survival (primary endpoint). We also found that overall survival (secondary endpoint) was similar with the two procedures. The results of post hoc exploratory analyzes examining the association between relevant clinical and demographic variables and disease-free and overall survival were consistent with the overall trial results.
However, given the small sample size and few events in each subgroup, these results should be interpreted with caution. Furthermore, no substantial differences were observed between the two groups in the incidences or patterns of disease recurrence. Locoregional recurrences were numerically slightly higher after sublobar resection than after lobectomy (13.4% vs. 10.0%), but the difference was not clinically significant. Although we did not require extension of lymph node dissection beyond sampling the major hilar stations and two mediastinal nodes, regional recurrence occurred in 1.8% of patients after sublobar resection and in 2. 6% of patients after lobectomy.
It is important that these results are interpreted strictly within the limitations of the eligibility criteria required by the trial.
(Funded by the National Cancer Institute and others; CALGB number 140503 ClinicalTrials.gov, NCT00499330. opens in new tab.)
















