Phase 3 Trial of Epicutaneous Immunotherapy in Children with Peanut Allergy
Background
There is no approved treatment for peanut allergy for children younger than 4 years, and the effectiveness and safety of epicutaneous immunotherapy with a peanut patch in young children with peanut allergy is unknown.
Methods
We conducted this phase 3, multicenter, double-blind, randomized, placebo-controlled trial involving children 1 to 3 years of age with peanut allergy confirmed by a double-blind, placebo-controlled food challenge.
Patients who had a trigger dose (the dose necessary to cause an allergic reaction) of 300 mg or less of peanut protein were assigned in a 2:1 ratio to receive epicutaneous immunotherapy administered via a peanut patch (group intervention) or to receive placebo administered daily for 12 months.
The primary endpoint was a response to treatment as measured by the trigger dose of peanut protein at 12 months. Safety was assessed according to the occurrence of adverse events during use of the peanut patch or placebo.
Results
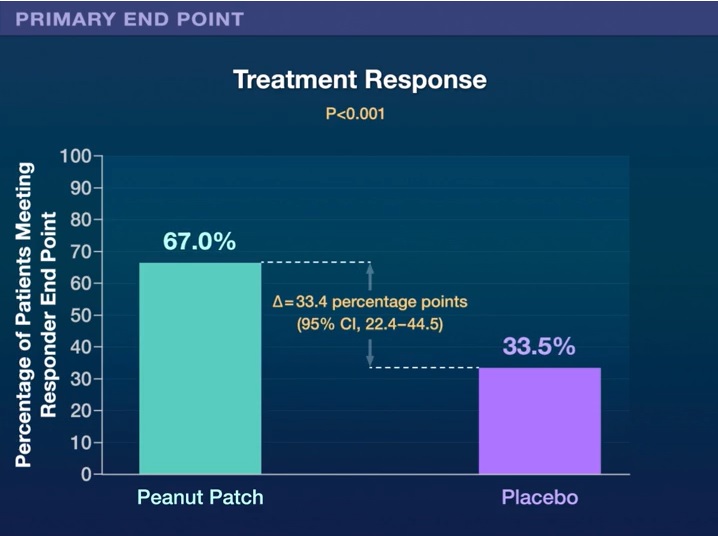
Of the 362 patients who were randomized, 84.8% completed the trial. The primary efficacy outcome was observed in 67.0% of children in the intervention group compared with 33.5% of those in the placebo group (risk difference, 33.4 percentage points; range 95% confidence, 22.4 to 44.5, P<0.001).
Adverse events that occurred during use of the intervention or placebo, regardless of the relationship, were observed in 100% of patients in the intervention group and 99.2% in the placebo group.
Serious adverse events occurred in 8.6% of patients in the intervention group and 2.5% of those in the placebo group; anaphylaxis occurred in 7.8% and 3.4%, respectively. Serious treatment-related adverse events occurred in 0.4% of patients in the intervention group and none in the placebo group.
Conclusions In this trial involving children aged 1 to 3 years with peanut allergy, epicutaneous immunotherapy for 12 months was superior to placebo in desensitizing children to peanuts and increasing the dose of peanut that triggered allergic symptoms. |
Comments
A global Phase 3 clinical trial found that a one-year immunotherapy via skin patch safely desensitized young children with peanut allergy, reducing the risk of a serious allergic reaction from accidental exposure.
A global Phase 3 clinical trial involving the Ann & Robert H. Lurie Children’s Hospital of Chicago found that a one-year immunotherapy via skin patch safely desensitizes young children with peanut allergy, reducing the risk of a serious allergic reaction from accidental exposure. The results of this randomized, double-blind, placebo-controlled trial for children ages 1 to 3 years, funded by DBV Technologies, were published in the New England Journal of Medicine .
"We were excited to contribute to this landmark study that holds so much promise for our young peanut allergy patients," said co-author Melanie Makhija, MD, who was the study’s principal investigator at Lurie Children’s and is an associate professor of Pediatrics at the College of Northwestern University Feinberg Medicine. "Children who originally reacted to a small fraction of peanuts were able to tolerate the equivalent of one to four peanuts after completing the treatment course. This means that these children will be well protected from accidental exposure to peanuts. Importantly, we found that "The peanut patch was safe, with very little chance of a serious allergic reaction. This is excellent news for families of children with peanut allergies."
Peanut allergy affects about 2 percent of children in the United States, Canada, and other Westernized countries, and commonly persists into adulthood. Life-threatening allergic reactions can be triggered by unintentional exposure to trace amounts, even through products manufactured on shared equipment such as peanuts. Currently, there are no approved treatments for children under 4 years of age who are allergic to peanuts.
Since 2012, Lurie Children’s food allergy clinical trials program has enrolled patients in numerous novel treatment studies, including oral immunotherapy for peanuts that has been approved by the Food and Drug Administration (FDA). Ongoing trials are available for all age groups, from infancy to adulthood. The program is led by principal investigators Elizabeth Lippner, MD, and Abigail Lang, MD, MSCI.
Research at Ann & Robert H. Lurie Children’s Hospital of Chicago is conducted through the Stanley Manne Children’s Research Institute. The Manne Research Institute is focused on improving children’s health, transforming pediatric medicine, and ensuring a healthier future through the relentless pursuit of knowledge. Lurie Children’s is a nonprofit organization committed to providing access to exceptional care for all children. It is ranked one of the best children’s hospitals in the country by US News & World Report. Lurie Children’s is the pediatric training center for Northwestern University Feinberg School of Medicine.
Reference : Phase 3 Trial of Epicutaneous Immunotherapy in Toddlers with Peanut Allergy. New England Journal of Medicine , 2023; 388 (19): 1755 DOI: 10.1056/NEJMoa2212895 Matthew Greenhawt, MD, Sayantani B. Sindher, MD, Julie Wang, MD, Michael O’Sullivan, et al.
(Funded by DBV Technologies; EPITOPE ClinicalTrials.gov number, NCT03211247. opens in new tab.)
















