Background
The ChAdOx1 nCoV-19 vaccine (AZD1222) has been approved for emergency use by the UK regulatory authority, Medicines and Healthcare products Regulatory Agency, with a regimen of two standard doses administered 4 to 12 weeks apart . The planned rollout in the UK will involve vaccinating people in high-risk categories with their first dose immediately and administering the second dose 12 weeks later.
Here, we provide an additional prespecified pooled analysis of ChAdOx1 nCoV-19 assays and exploratory analyzes of the impact on immunogenicity and efficacy of extending the interval between priming and booster doses. Furthermore, we show the immunogenicity and protection provided by the first dose , before a booster dose is offered.
Methods
We present data from three single-blind randomized controlled trials: a phase 1/2 study in the United Kingdom (COV001), a phase 2/3 study in the United Kingdom (COV002), and a phase 3 study in Brazil (COV003). , and a double-blind phase 1/2 study in South Africa (COV005).
As described above, individuals aged 18 years or older were randomized 1:1 to receive two standard doses of ChAdOx1 nCoV-19 (5 × 1010 viral particles) or a control vaccine or a saline placebo. In the UK trial, a subset of participants received a lower dose (2 2 × 1010 viral particles) of ChAdOx1 nCoV-19 for the first dose.
The primary outcome was virologically confirmed symptomatic COVID-19 disease, defined as a positive swab nucleic acid amplification test (NAAT) combined with at least one qualifying symptom (fever ≥378°C, cough, shortness of breath, or anosmia or ageusia) more than 14 days after the second dose.
Secondary efficacy analyzes included cases that occurred at least 22 days after the first dose. Antibody responses measured by immunoassay and by pseudovirus neutralization were exploratory results.
All NAAT swab-positive COVID-19 cases were adjudicated for inclusion in the analysis by a blinded independent parameter review committee.
The primary analysis included all participants who were seronegative for the SARS-CoV-2 N protein at baseline, had at least 14 days of follow-up after the second dose, and had no evidence of prior SARS-CoV-2 infection. 2 per NAAT swabs.
Safety was assessed in all participants who received at least one dose. The four trials are registered with ISRCTN89951424 (COV003) and ClinicalTrials.gov, NCT04324606 (COV001), NCT04400838 (COV002) and NCT04444674 (COV005).
Results
Between April 23 and December 6, 2020, 24,422 participants were recruited and vaccinated across the four studies, of whom 17,178 were included in the primary analysis (8,597 received ChAdOx1 nCoV-19 and 8,581 received the control vaccine). The data cutoff for these analyzes was December 7, 2020. 332 NAAT-positive infections met the primary endpoint of symptomatic infection more than 14 days after the second dose.
The overall efficacy of the vaccine more than 14 days after the second dose was 66% (95% CI: 57 4-74 0), with 84 (10%) cases in the 8597 participants in the ChAdOx1 nCoV-19 group and 248 (2 9%) in the 8581 control group participants.
There were no hospital admissions for COVID-19 in the ChAdOx1 nCoV-19 group after the initial 21-day exclusion period, and 15 in the control group. 108 (0.9%) of 12,282 participants in the ChAdOx1 nCoV-19 group and 127 (11%) of 11,962 participants in the control group had serious adverse events.
There were seven deaths considered unrelated to vaccination (two in the ChAdOx1 nCov-19 group and five in the control group), including one COVID-19-related death in a control group participant.
Exploratory analyzes showed that vaccine efficacy after a single standard dose of vaccine from day 22 to day 90 after vaccination was 76.0% (59.3–85.9). Our modeling analysis indicated that protection did not decline during this initial 3-month period.
Similarly, antibody levels were maintained during this period with minimal decline by day 90 (geometric mean ratio [GMR] 0·66 [95% CI 0·59-0·74]).
In participants who received two standard doses, after the second dose, efficacy was greater in those with a longer priming interval (vaccine efficacy 81.3% [95% CI 60.3–91.2] at ≥12 weeks) than in those with a short interval (vaccine efficacy 55·1% [33·0–69·9] in <6 weeks).
These observations are supported by immunogenicity data that showed binding antibody responses more than doubled after an interval of 12 or more weeks compared to an interval of less than 6 weeks in those aged 18 to 55 years (GMR 2 32 [2·01–2·68]).
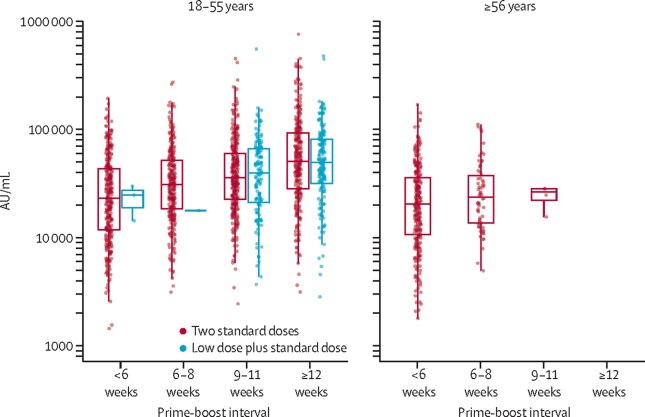
Peak anti-SARS-CoV-2 IgG responses by multiplex immunoassay at 28 days after second dose in participants receiving two standard doses or low dose plus standard dose, by prime-boost interval (n = 3337)
Interpretation The results of this primary analysis of two doses of ChAdOx1 nCoV-19 were consistent with those observed in the interim analysis of the trials and confirm that the vaccine is effective, with results varying by dose interval in the exploratory analyses. A 3-month dosing interval could have advantages over a short dosing interval schedule for pandemic vaccine rollout to protect as many people in the population as quickly as possible when supplies are tight, while at the same time time improve protection after receiving a second dose. |
Research in context
Evidence before this study
The ChAdOx1 nCoV-19 vaccine (AZD1222) was approved for emergency use authorization in the United Kingdom based on interim efficacy results from 131 cases of primary symptomatic COVID-19, with efficacy based on two of the four trials of the vaccine. The planned rollout of the vaccine in the UK involves administering two doses 12 weeks apart, a policy that has received substantial feedback.
Added value of this study
This report provides updated primary efficacy results after an additional month of data collection. The interim report included 131 cases of primary symptomatic COVID-19. The latest results with additional follow-up include 332 cases of primary symptomatic COVID-19. The efficacy estimates now include data from all four studies of the vaccine from three countries, while the interim analysis included only two studies in the efficacy assessments due to the small number of cases in the smaller studies.
In addition to the primary evaluation of efficacy, post-hoc exploratory analyzes have been added, including a breakdown of efficacy by prime-boost interval and the efficacy of a single dose of vaccine.
Implications of all available evidence
The primary analysis supports the findings reported in the interim analysis that the vaccine is effective and safe.
Exploratory analyzes show that greater vaccine efficacy is obtained with a longer booster interval , and that a single dose of vaccine is effective in the first 90 days, providing further evidence for current policy.
Vaccination programs aimed at vaccinating a large proportion of the population with a single dose, with a second dose administered after a period of 3 months , could be an effective strategy for reducing disease and could have advantages over a program with a short initial window for rollout of a pandemic vaccine when supplies are short in the short term. Two doses of ChAdOx1 nCoV-19 were effective in preventing symptomatic COVID-19. These results confirm those observed in the interim analysis of the trials. |