The STEP-HFpEF trial was presented in a Hot Line session at the ESC 2023 Congress
Weight Loss Drugs Benefit Heart Failure and Obesity Patients
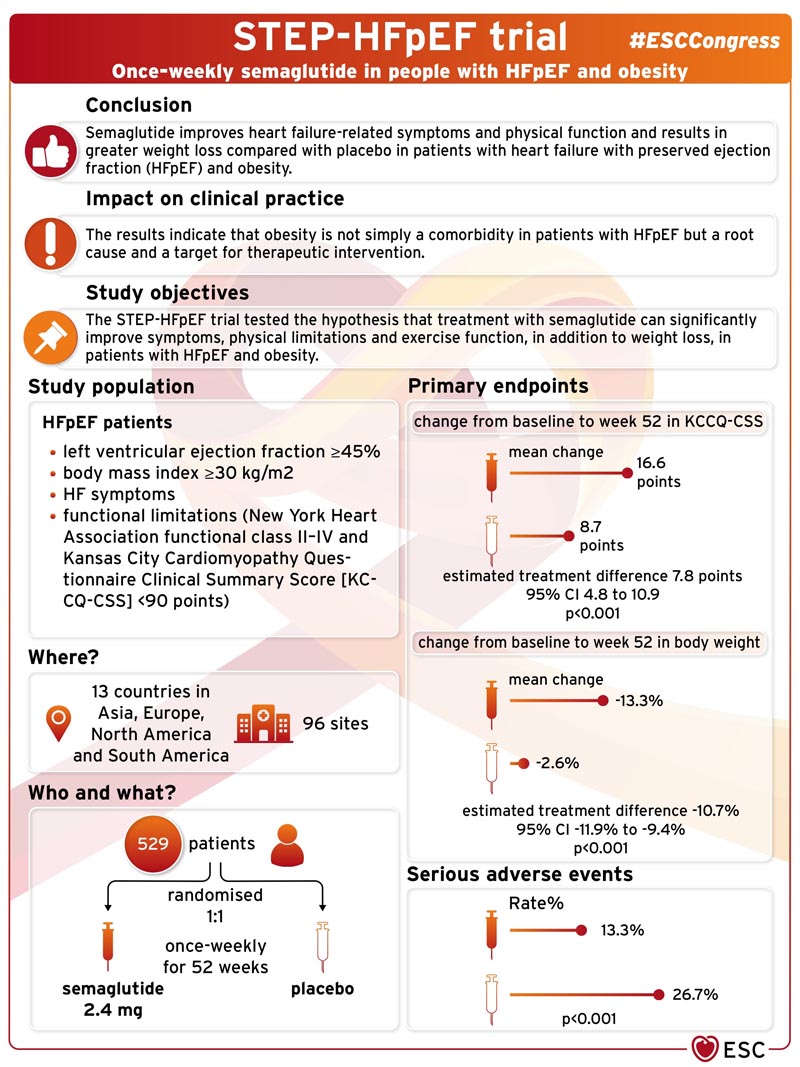
Semaglutide improves heart failure -related symptoms and physical function and produces greater weight loss compared with placebo in patients with heart failure with preserved ejection fraction (HFpEF) and obesity, according to breaking research presented in a Hot Line Session at the ESC 2023 Congress.

Approximately half of heart failure patients in the community have heart failure with preserved ejection fraction (HFpEF). The majority of patients with HFpEF are overweight or obese , and growing evidence suggests that obesity and excess adiposity are not simply comorbidities, but may play a critical role in the development and progression of HFpEF. Patients with obesity-related HFpEF have an especially high burden of debilitating symptoms (shortness of breath, exertion intolerance, swelling/edema) and physical limitations, which together result in poor quality of life. There are few treatment options available and there are no approved therapies specifically targeting the obesity phenotype of HFpEF.
Semaglutide is a potent glucagon-like peptide-1 receptor agonist that has previously been shown to produce substantial weight loss in overweight and obese people . The STEP-HFpEF trial tested the hypothesis that semaglutide treatment can significantly improve symptoms, physical limitations, and exercise function, in addition to weight loss, in patients with HFpEF and obesity.
STEP-HFpEF was a randomized, double-blind, placebo-controlled trial conducted at 96 centers in 13 countries in Asia, Europe, North America, and South America. The trial included patients with HFpEF (left ventricular ejection fraction ≥45%), body mass index (BMI) ≥30 kg/m2, symptoms of heart failure, and functional limitations (New York Heart Association functional class II-IV and Kansas City Cardiomyopathy Questionnaire Clinical Summary Score [KCCQ-CSS] <90 points).
Participants were randomly assigned in a 1:1 ratio to receive 2.4 mg of subcutaneous semaglutide once a week or placebo for 52 weeks .
The trial had two primary endpoints : change from baseline to week 52 in 1) KCCQ-CSS, a gold standard measure of heart failure-related symptoms and physical limitations; and 2) body weight. Confirmatory secondary endpoints included change in 6-minute walk distance (6MWD, a validated measure of exercise function); a hierarchical composite endpoint of death, heart failure events, and change in KCCQ-CSS and 6MWD; and change in C-reactive protein (CRP, a measure of inflammation).
The trial included 529 patients . The median age was 69 years and 56.1% were women. Median body weight and BMI at baseline were 105.1 kg and 37.0 kg/m2, respectively. At baseline, patients had a substantial degree of heart failure-related symptoms, physical limitations, and poor exercise tolerance: 66.2% were NYHA class II and 33.8% were NYHA class III-IV. the NYHA; the median of the KCCQ-CSS was 58.9 points; and the median 6MWD was 320 meters.
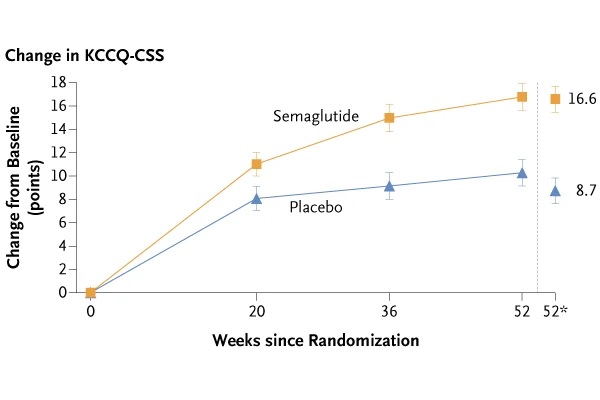
The trial met both primary endpoints and all confirmatory secondary endpoints. The mean change in KCCQ-CSS from baseline to week 52 was 16.6 points with semaglutide versus 8.7 points with placebo (estimated treatment difference [ETD]: 7.8 points, confidence interval [CI] 95%: 4.8 to 10.9; p<0.001). The mean change in body weight from baseline to week 52 was -13.3% with semaglutide versus -2.6% with placebo (ETD: -10.7%, 95% CI: -11.9% to - 9.4%; p<0.001).
Regarding secondary endpoints, the mean change in 6-minute walk (6MWD) was 21.5 meters for semaglutide versus 1.2 meters for placebo (ETD: 20.3 meters, 95% CI: 8 .6 to 32.1; p<0.001). For the hierarchical composite endpoint, semaglutide produced more wins than placebo (win ratio 1.72, 95% CI 1.37 to 2.15, p<0.001). The mean change in CRP was -43.5% and -7.3% with semaglutide and placebo, respectively (estimated treatment ratio 0.61, 95% CI 0.51 to 0.72, p<0.001 ).
In terms of exploratory endpoints, the change in NTproBNP at 52 weeks was -20.9% and -5.3% for semaglutide versus placebo (estimated treatment ratio 0.84, 95% CI 0.71 to 0.98). One patient in the semaglutide group and 12 in the placebo group experienced an adjudicated event of heart failure hospitalization or urgent visit (hazard ratio 0.08, 95% CI 0.00 to 0.42).
Serious adverse events were reported in 35 (13.3%) and 71 (26.7%) participants with semaglutide and placebo, respectively (p<0.001).
Lead researcher Dr Mikhail Kosiborod from Saint Luke’s Mid America Heart Institute, Kansas City, USA, said: "In patients with HFpEF and obesity, treatment with 2.4 mg of semaglutide produced large improvements in symptoms , physical limitations and exercise function, reduced inflammation and resulted in greater weight loss and fewer serious adverse events compared to placebo. To our knowledge, this is the first trial of a pharmacological agent specifically targeting obesity as a treatment strategy for HFpEF, and the magnitude of benefit we observed is the greatest seen with any agent in HFpEF. This is likely to have a significant impact on clinical practice, especially as there is a paucity of effective therapies in this group of vulnerable patients.
Conclusions In patients with heart failure with preserved ejection fraction and obesity, treatment with semaglutide (2.4 mg) produced greater reductions in symptoms and physical limitations, greater improvements in exercise function, and greater weight loss than placebo. |
: Reference : Semaglutide in Patients with Heart Failure with Preserved Ejection Fraction and Obesity . Mikhail N. Kosiborod, MD, Steen Z. Abildstrøm, Ph.D., Barry A. Borlaug, MD, Javed Butler, MD, et al. NEJM DOI: 10.1056/NEJMoa2306963
(Funded by Novo Nordisk; STEP-HFpEF ClinicalTrials.gov number, NCT04788511. opens in new tab.)
















