Background
The effectiveness of inhaled glucocorticoids in shortening the time to resolution of symptoms or preventing hospitalization or death among outpatients with mild to moderate coronavirus disease 2019 (Covid-19) is unclear.
Methods
We conducted a decentralized, double-blind, randomized, placebo-controlled platform trial in the United States to evaluate the use of repurposed medications in outpatients with confirmed coronavirus disease 2019 (Covid-19).
Non-hospitalized adults aged 30 years or older who had at least two symptoms of acute infection that had been present for no more than 7 days before enrollment were randomly assigned to receive inhaled fluticasone furoate at a dose of 200 μg. once a day for 14 days or placebo.
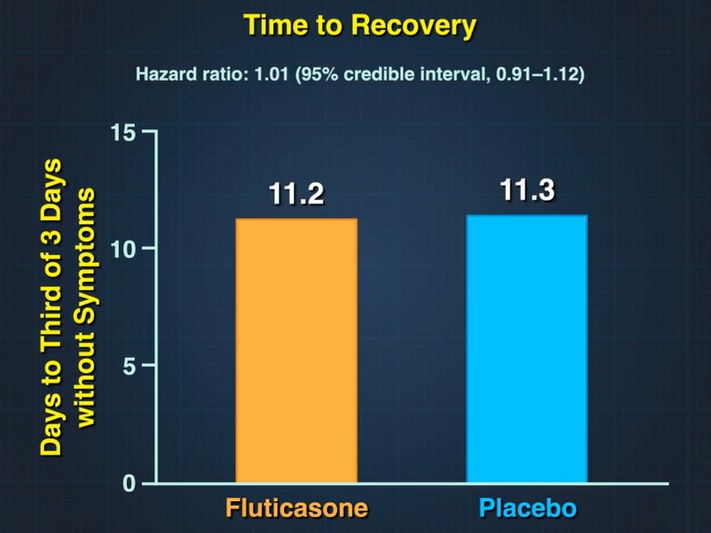
The primary outcome was time to sustained recovery, defined as the third of 3 consecutive symptom-free days. Key secondary outcomes included hospitalization or death through day 28 and a composite outcome of need for an emergency or emergency department visit or hospitalization or death through day 28.
Results
Of the 1,407 enrolled participants who underwent randomization, 715 were assigned to receive inhaled fluticasone furoate and 692 to receive placebo, and 656 and 621, respectively, were included in the analysis.
There was no evidence that fluticasone furoate use resulted in a shorter recovery time than placebo (hazard ratio, 1.01; 95% credible interval, 0.91 to 1.12; posterior probability of benefit [defined as a risk ratio >1], 0.56).
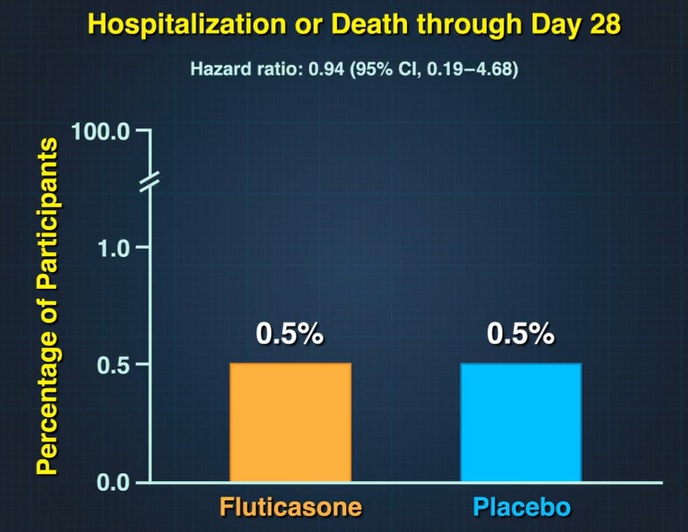
A total of 24 participants (3.7%) in the fluticasone furoate group had emergency department visits or were hospitalized, compared with 13 participants (2.1%) in the placebo group (hazard ratio , 1.9; 95% credibility interval). 0.8 to 3.5).
Three participants in each group were hospitalized and no deaths occurred. Adverse events were rare in both groups.
Conclusions Treatment with inhaled fluticasone furoate for 14 days did not result in a shorter recovery time than placebo among outpatients with Covid-19 in the United States. |
(Funded by the National Center for Advancing Translational Sciences and others; ACTIV-6 ClinicalTrials.gov number, NCT04885530. opens in a new tab.)