The Nobel Assembly at the Karolinska Institute today decided to award the 2023 Nobel Prize in Physiology or Medicine jointly to Katalin Karikó and Drew Weissman for their discoveries on nucleoside base modifications that enabled the development of effective mRNA vaccines against COVID-19.
The discoveries of the two Nobel laureates were instrumental in developing effective mRNA vaccines against COVID-19 during the pandemic that began in early 2020. Through their groundbreaking discoveries, which have fundamentally changed our understanding of how mRNA interacts with our immune system, the honorees contributed to the unprecedented rate of vaccine development during one of the greatest threats to human health in modern times.
Vaccines before the pandemic
Vaccination stimulates the formation of an immune response to a particular pathogen. This gives the body an advantage in fighting disease in the event of subsequent exposure. Vaccines based on killed or weakened viruses have long been available, such as vaccines against polio, measles and yellow fever. In 1951, Max Theiler received the Nobel Prize in Physiology or Medicine for developing the yellow fever vaccine.
Thanks to advances in molecular biology in recent decades, vaccines have been developed based on individual viral components, rather than whole viruses. Parts of the viral genetic code, which typically encode proteins found on the surface of the virus, are used to produce proteins that stimulate the formation of virus-blocking antibodies. Some examples are vaccines against hepatitis B virus and human papillomavirus. Alternatively, parts of the viral genetic code can be moved into a harmless carrier virus, a “vector.” This method is used in vaccines against the Ebola virus. When vector vaccines are injected, the selected viral protein is produced in our cells, which stimulates an immune response against the target virus.
The production of vaccines based on whole viruses, proteins and vectors requires large-scale cell culture. This resource-intensive process limits the possibilities for rapid vaccine production in response to outbreaks and pandemics. Therefore, researchers have long attempted to develop cell culture-independent vaccine technologies, but this has proven to be challenging.
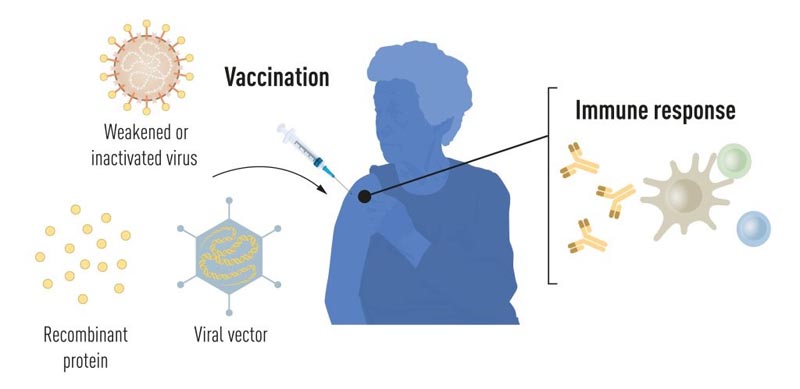
Figure 1. Vaccine production methods before the COVID-19 pandemic .
mRNA vaccines: a promising idea
In our cells, the genetic information encoded in DNA is transferred to messenger RNA (mRNA), which is used as a template for protein production. Efficient methods to produce mRNA without cell culture, called in vitro transcription, were introduced during the 1980s. This decisive step accelerated the development of molecular biology applications in various fields. Ideas of using mRNA technologies for therapeutics and vaccines also took off, but there were still obstacles ahead. In vitro transcribed mRNA was considered unstable and difficult to deliver, requiring the development of sophisticated carrier lipid systems to encapsulate the mRNA. Furthermore, in vitro produced mRNA gave rise to inflammatory reactions. Therefore, enthusiasm for developing mRNA technology for clinical purposes was initially limited.
These obstacles did not discourage Hungarian biochemist Katalin Karikó, who dedicated herself to developing methods to use mRNA for therapeutic purposes. In the early 1990s, when she was an assistant professor at the University of Pennsylvania, she remained true to her vision of realizing mRNA as a therapeutic despite encountering difficulties convincing research funders of the importance of her project. . A new colleague of Karikó at her university was immunologist Drew Weissman. He was interested in dendritic cells, which have important roles in immune surveillance and activation of vaccine-induced immune responses. Spurred by new ideas, a fruitful collaboration between the two soon began, focusing on how different types of RNA interact with the immune system.
The great advance
Karikó and Weissman observed that dendritic cells recognize mRNA transcribed in vitro as a foreign substance, leading to their activation and the release of inflammatory signaling molecules. They wondered why mRNA transcribed in vitro was recognized as foreign, while mRNA from mammalian cells did not give rise to the same reaction. Karikó and Weissman realized that some critical properties must distinguish different types of mRNA.
RNA contains four bases, abbreviated A, U, G and C, which correspond to A, T, G and C in DNA, the letters of the genetic code. Karikó and Weissman knew that the bases of RNA from mammalian cells are often chemically modified, while mRNA transcribed in vitro is not. They wondered whether the absence of altered bases in the in vitro transcribed RNA could explain the unwanted inflammatory reaction.
To investigate this, they produced different mRNA variants, each with unique chemical alterations in their bases, which they delivered to dendritic cells. The results were surprising: the inflammatory response was almost abolished when base modifications were included in the mRNA. This was a paradigm shift in our understanding of how cells recognize and respond to different forms of mRNA. Karikó and Weissman immediately understood that their discovery had profound importance for the use of mRNA as therapy. These pivotal results were published in 2005, fifteen years before the COVID-19 pandemic.

Figure 2 . mRNA contains four different bases, abbreviated A, U, G and C. Nobel laureates discovered that base-modified mRNA can be used to block the activation of inflammatory reactions (secretion of signaling molecules) and increase protein production when The mRNA is delivered to the cells.
In additional studies published in 2008 and 2010, Karikó and Weissman demonstrated that delivery of mRNA generated with base modifications markedly increased protein production compared to unmodified mRNA. The effect was due to reduced activation of an enzyme that regulates protein production. Thanks to their discoveries that base modifications reduced inflammatory responses and increased protein production, Karikó and Weissman had removed critical obstacles on the path to clinical applications of mRNA.
mRNA vaccines discovered their potential
Interest in mRNA technology began to increase, and by 2010, several companies were working on developing the method. Vaccines against the Zika virus and MERS-CoV were sought; the latter is closely related to SARS-CoV-2. After the outbreak of the COVID-19 pandemic, two mRNA vaccines with modified bases encoding the surface protein of SARS-CoV-2 were developed at a record pace. Protective effects of around 95% were reported and both vaccines were approved as early as December 2020.
The impressive flexibility and speed with which mRNA vaccines can be developed pave the way for using the new platform for vaccines against other infectious diseases as well. In the future, the technology could also be used to deliver therapeutic proteins and treat some types of cancer.
Other SARS-CoV-2 vaccines, based on different methodologies, were also rapidly introduced, and together more than 13 billion COVID-19 vaccine doses have been administered worldwide. Vaccines have saved millions of lives and prevented serious illness in many more, allowing societies to open up and return to normal conditions. Through their fundamental discoveries on the importance of base modifications in mRNA, this year’s Nobel Laureates made a fundamental contribution to this transformative development during one of the greatest health crises of our time.
Key publications
- Karikó, K., Buckstein, M., Ni, H. and Weissman, D. Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity 23, 165-175 (2005).
- Karikó, K., Muramatsu, H., Welsh, FA, Ludwig, J., Kato, H., Akira, S. and Weissman, D. Incorporation of pseudouridine into mRNA produces a superior non-immunogenic vector with greater translational capacity and biological stability. Mol Ther 16, 1833–1840 (2008).
- Anderson, BR, Muramatsu, H., Nallagatla, SR, Bevilacqua, PC, Sansing, LH, Weissman, D. and Karikó, K. Incorporation of pseudouridine into mRNA enhances translation by decreasing PKR activation. Nucleic acids Res. 38, 5884–5892 (2010).

Biographies
Katalin Karikó was born in 1955 in Szolnok, Hungary. She received her PhD from the University of Szeged in 1982 and conducted postdoctoral research at the Hungarian Academy of Sciences in Szeged until 1985. She then conducted postdoctoral research at Temple University, Philadelphia, and the University of Health Sciences, Bethesda. In 1989, she was appointed assistant professor at the University of Pennsylvania, where she remained until 2013. After that, she became vice president and then senior vice president of BioNTech RNA Pharmaceuticals. As of 2021, she is a professor at the University of Szeged and an adjunct professor at the Perelman School of Medicine at the University of Pennsylvania.
Drew Weissman was born in 1959 in Lexington, Massachusetts, USA. He received his MD and PhD from Boston University in 1987. He completed his clinical training at Beth Israel Deaconess Medical Center at Harvard Medical School and conducted research postdoctoral fellows at the National Institutes of Health. In 1997, Weissman established his research group at the Perelman School of Medicine at the University of Pennsylvania. He is the Roberts Family Professor in Vaccine Research and director of the Penn Institute for RNA Innovations.
Illustrations: © Nobel Committee for Physiology or Medicine. Illustrator: Mattías Karlén
The Nobel Assembly, made up of 50 professors from the Karolinska Institute, awards the Nobel Prize in Physiology or Medicine. Its Nobel Committee evaluates the nominations. Since 1901, the Nobel Prize has been awarded to scientists who have made the most important discoveries for the benefit of humanity.