Researchers from the Precision Healthcare University Research Institute (PHURI) at Queen Mary University of London and the Berlin Institute of Health (BIH) at Charité – Universitätsmedizin Berlin have identified the genetic causes of Raynaud’s phenomenon . Their findings, published in Nature Communications , could lead to the first effective treatments for people with Raynaud’s disease.
Raynaud’s phenomenon ( RP) is an inherited condition that affects blood circulation. It is a vasospastic condition, meaning that small blood vessels near the surface of the skin spasm that can limit blood flow. People with Raynaud’s often experience pain in their fingers and toes, often along with skin color changes, due to a lack of blood flow during attacks when they are cold or emotionally stressed. In more severe cases, it can cause severe pain or ulcers.
About 2-5% of the population is affected by Raynaud’s disease.
Despite being a common condition, it is under-researched and little is known about the genetic cause of the condition. There are limited treatments available for RP. Doctors often advise the patient to use "self-care" strategies , such as staying warm and avoiding attack triggers. In severe cases, medications, which are "repurposed," may be prescribed , usually medications to reduce high blood pressure. These often cause serious side effects in patients. A better understanding of the underlying genetic mechanisms that cause RD is needed to develop safe and effective treatments.
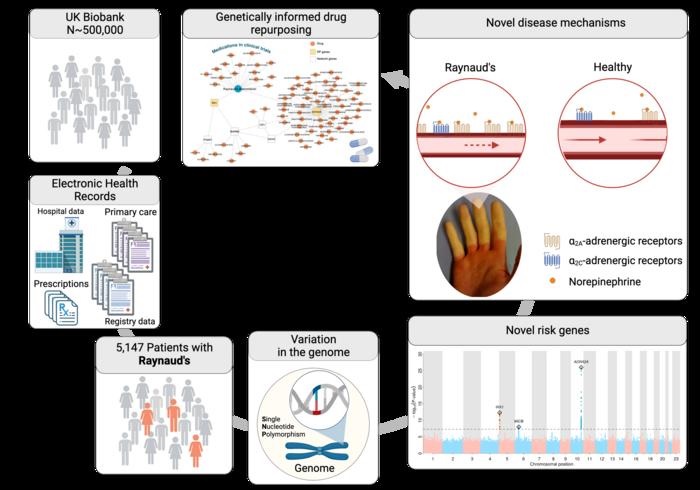
Researchers led by Professor Claudia Langenberg and Professor Maik Pietzner, working at PHURI and BIH, carried out the largest genetic study of Raynaud’s phenomenon. The team used electronic medical records from the UK Biobank, a large-scale biomedical database and research resource containing genetic and health information on half a million UK participants, to identify more than 5,000 people affected by Raynaud’s disease. The team also used electronic medical records from Queen Mary’s Genes & Health study.
The results
The researchers discovered variations in two genes that predisposed participants to Raynaud’s phenomenon: one was the alpha-2A-adrenergic receptor for adrenaline, ADRA2A, a classic stress receptor that causes small blood vessels to constrict.
“This makes sense when it is cold or dangerous, because the body has to supply blood inside the body,” explains Maik Pietzner, professor of health data modeling at PHURI and group leader at BIH.
"In Raynaud’s patients, this receptor seemed to be particularly active, which could explain vasospasms , especially in combination with the second gene we found: this gene is the transcription factor IRX1, which can regulate the ability of vessels to dilate. blood.”
“If its production is increased, it can activate genes that prevent constricted vessels from relaxing as they normally would. This, along with the overactive adrenaline receptor , can cause the blood vessels to not supply enough blood for a longer period of time, causing white fingers and toes to be seen.”
The researchers replicated some of their findings using data from participants of British, Bangladeshi and Pakistani origin from the Queen Mary’s Genes & Health study. The researchers’ findings help to understand for the first time why small vessels react so strongly in patients, even apparently without external stimuli, such as exposure to cold.
Dr Emma Blamont, Head of Scleroderma and Raynaud’s Research UK (SRUK), said: "Raynaud’s disease is a chronic, painful disease that affects around one in six people in the UK. We know "Certain triggers such as cold and stress can cause attacks, but relatively little is known about why some people experience Raynaud’s disease and others do not." For the millions of people living with this condition, simple everyday tasks can be a challenge, so research like this, which significantly advances our understanding of Raynaud’s disease and the role genetics may play in its cause, it’s crucial."
“The next step is to confirm these important findings in more diverse population groups and validate the results through functional studies. "If successful, these findings could help us unlock more new therapeutic avenues for Raynaud’s leading to better, more specific and gentler treatments."
The findings could lead to recommendations for patients to help manage the condition or its symptoms. For example, researchers have shown that people with a genetic predisposition to low blood sugar have a higher risk of Raynaud’s phenomenon, suggesting that patients should possibly avoid longer episodes of low blood sugar. .
For Claudia Langenberg, director of PHURI and professor of Computational Medicine at BIH, this study exemplifies that the integration of data from genomic and electronic medical records can quickly help to better understand diseases whose etiology remains unknown. “Of course, ultimately we hope that our findings will point to new treatment options."There are already approved medications that more or less specifically inhibit ADRA2A function, such as the antidepressant mirtazapine , and our results suggest that they may present alternative treatment options for patients suffering from the symptoms of Raynaud’s disease."















