
American Heart Association Scientific Sessions 2023, Latest Scientific Summary LBS.04
Research Highlights:
|
Zilebesiran is an investigational RNA interference agent that targets angiotensinogen (AGT), a hormone produced predominantly in the liver that contributes to blood pressure regulation.
“Uncontrolled high blood pressure is a leading cause of death and disease, so new treatments are needed that provide sustained blood pressure control over longer periods of time. This will improve outcomes for people with hypertension,” said the study’s senior author, George L. Bakris, MD, FAHA, professor of medicine and director of the Comprehensive Hypertension Center at the University of Chicago Medicine. "Uncontrolled hypertension also increases the risk of heart disease."
According to the American Heart Association’s 2023 Statistical Update, heart disease remains the leading cause of death in the United States and nearly half of adults in the country have high blood pressure.
In this global, randomized, double-blind, placebo-controlled trial, researchers investigated the safety and effectiveness of giving zilebesiran to people with mild to moderate high blood pressure, defined as systolic blood pressure of 135 to 160 mm Hg, which are without treatment or in stable therapy with up to two antihypertensive medications. Systolic blood pressure is the top number in a blood pressure reading and indicates how much pressure the blood exerts against the walls of the arteries when the heart contracts.
The 2017 ACC/AHA Guideline for the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure in Adults classifies stage 1 hypertension as those with upper and lower blood pressure measurements greater than or equal to 130/80 mm Hg, and stage 2 hypertension as having upper and lower measurements greater than or equal to 140/90 mm Hg.
The study included 394 patients with an average systolic blood pressure of 142 mm Hg. Participants were randomly assigned to receive subcutaneous doses of zilebesiran (four groups: 150, 300, or 600 mg once every six months or 300 mg once every three months) or a placebo.
Over the six-month study period, the analysis found:
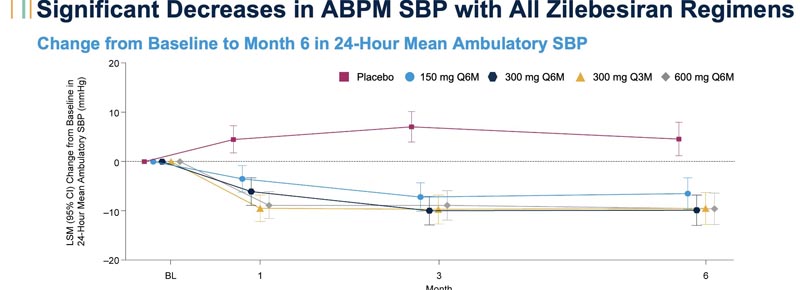
Compared with participants receiving placebo, participants receiving single doses of zilebesiran had reductions greater than 10 mm Hg, on average, in 24-hour systolic blood pressure and reductions greater than 90% in serum AGT levels, a hormone produced predominantly in the liver that contributes to the regulation of blood pressure.
At three-month follow-up, participants in the 300 mg and 600 mg zilebesiran groups had average 24-hour systolic blood pressure reduced by 15 mm Hg or more on average.
After six months, people receiving zilebesiran were significantly more likely to experience average 24-hour systolic blood pressure reductions of 20 mm Hg or more on average without taking additional high blood pressure medications.
People who received zilebesiran were more likely to achieve average 24-hour systolic blood pressure measurements of 130 mm Hg or less at six months.
Additionally, participants in all four zilebesiran groups consistently experienced significantly greater reductions in both daytime and nighttime systolic blood pressure. Low rates of adverse events related to zilebesiran have been reported, with the most common being mild injection site reactions. Bakris noted that no clinically relevant changes in kidney or liver function were observed.
"Our study demonstrates that quarterly or semiannual doses of zilebesiran can effectively and safely reduce blood pressure in patients with uncontrolled hypertension," Bakris said. “It is well known that reductions in systolic blood pressure greater than or equal to 5 mm Hg are related to a reduction in cardiovascular risk. “These results reinforce the potential of zilebesiran to provide sustained blood pressure control, improve medication adherence through infrequent dosing, and in turn improve outcomes for people with high blood pressure.”
Study details and background:
394 patients were randomized and 377 patients were ultimately included in the analysis: 302 were randomly assigned to one of the four zilebesiran dosing groups and 75 people to the placebo group. Their average age was 57 years.
About 25% of participants were black adults; 56% were men.
The study was conducted at sites in Canada, Ukraine, the UK, and the US, with 78% of participants living in the US due to challenges with data collection due to the war in Ukraine. , data from the 16 patients randomized in Ukraine were excluded. of the analyses.
The average blood pressure of participants at study enrollment was 141.8/81.8 mm Hg.
There were four related non-serious adverse reactions leading to discontinuation in the zilebesiran groups: two cases of orthostatic hypotension; one of raising blood pressure; and one reaction at the injection site. There were no adverse reactions in the placebo group.
The six-month placebo-controlled treatment period of the Phase 2 trial reported here was conducted from July 2021 to June 2023.
These findings are limited because the KARDIA-1 trial evaluated zilebesiran only in people with mild to moderate hypertension. Another limitation of the study is that the results are limited to the six-month placebo-controlled period. Future research will evaluate long-term safety and impact on cardiovascular outcomes.
















