Summary table: - Large randomized trials have demonstrated high efficacy of the Oxford-AstraZeneca and PfizerBioNTech vaccines against laboratory-confirmed symptomatic SARS-CoV-2 infection. - The real-world effectiveness of these vaccines against any SARS-CoV-2 infection, including those without symptoms, is less clear, especially among the elderly who were underrepresented in the Oxford-AstraZeneca trial. What this study adds - SARS-CoV-2 infections decrease substantially after a first dose of either vaccine; Two doses of the Pfizer-BioNTech vaccine provided even greater protection, to a similar degree as prior infection with SARS-CoV2. - Vaccination and prior infection were most effective in reducing symptomatic infections and infections with high viral load, with smaller reductions in infections that do not cause symptoms and with lower viral load. - Both vaccines appear to be very effective against infections compatible with B.1.1.7 |
Goals
To evaluate the effectiveness of the COVID-19 vaccine in preventing SARS-CoV-2 infection in the community.
Design
Prospective cohort study.
Around
The UK population-representative longitudinal survey of COVID-19 infection.
Participants
373,402 participants aged 16 years and older who contributed 1,610,562 RT-PCR results from nose and throat swabs between December 1, 2020 and April 3, 2021.
Main outcome measures
New SARS-CoV-2 RT-PCR-positive episodes overall, by self-reported symptoms, by cycle threshold (Ct) value (<30 versus ≥30), and by genetic positivity (compatible with B.1.1.7 variant versus not).
Results
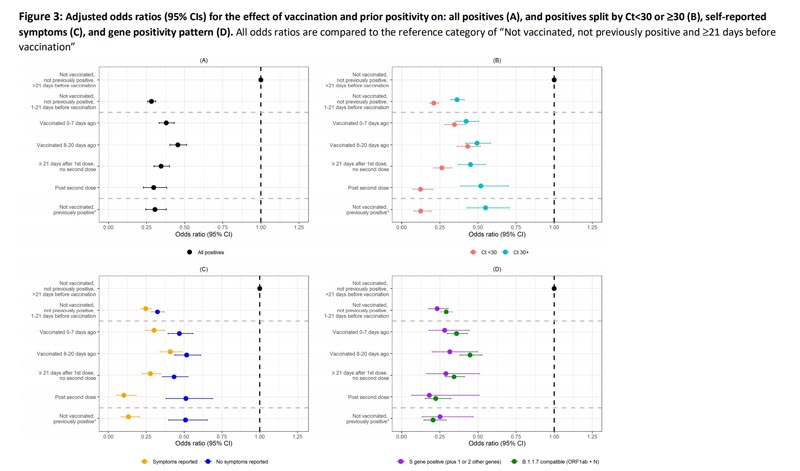
The odds of a new SARS-CoV-2 infection were reduced by 65% (95% CI: 60% to 70%; P < 0.001) in the ≥21 days from first vaccination without a second dose compared with unvaccinated individuals without evidence of previous infection (RT-PCR or antibody).
In the vaccinated, the greatest reduction in odds was observed after the second dose ( 70% , 95% CI: 62 to 77%; P < 0.001).
There was no evidence that these benefits varied between the Oxford-AstraZeneca and Pfizer-BioNTech vaccines (P > 0.9).
There was no evidence of a difference in the odds of a new SARS-CoV-2 infection for people who had received two doses of vaccine and with evidence of previous infection but not vaccinated (P = 0.89).
Vaccination had a greater impact on reducing SARS-CoV-2 infections with evidence of high viral shedding Ct < 30 (88% reduction after two doses; 95% CI: 80% to 93%; P < 0.001) and with self-reported symptoms % reduction after two doses; 95% CI: 82 to 94%; P < 0.001); the effects were similar for different patterns of genetic positivity.
Discussion
Main findings
- Results from this large community surveillance study show that COVID-19 vaccination significantly reduced the odds of people testing positive by PCR with a new SARS-CoV-2 infection, with greater reductions in new infections with Ct < 30 and self-reported symptoms, and in those who had received 2 doses of vaccine.
- The reductions provided by vaccination were similar to those provided by natural immunity.
- The protective effect of vaccination was attenuated in infections with Ct ≥ 30 and without self-reported symptoms.
- There was no evidence of any difference in effectiveness between the Pfizer-BioNTech and Oxford-AstraZeneca vaccines, or in those with long-term health problems.
- We observed greater reductions in new infections in those over 75 years of age compared to those under 75 years of age.
Highlights:
- A large real-world effectiveness study based on UK National Statistics Office data shows a large reduction in post-vaccine positivity after a single dose. A reduction of 65% (CI 60%, 70%) is observed, with no important differences by type of vaccine.
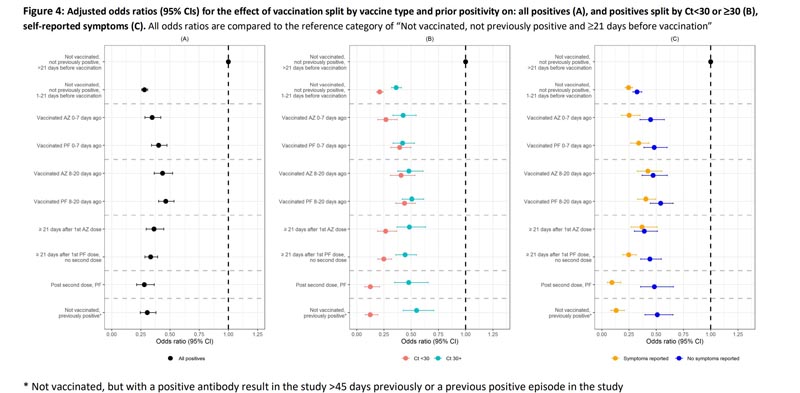
- There is a slight increase in effectiveness after the second dose at 70% (CI 62%, 77%) but not enough to be significant. However, there is a much greater reduction in the severity of infection, suggesting important transmission benefits. For CT <30, it is set to 88% (80%, 93%).
- For self-reported symptoms, it is similarly at 90% (82%, 94%).
- By age, there was a slightly greater benefit for those over 75 years of age with an effectiveness of 76% (68%, 82%), compared to 62% (56%, 67%) for those under 75 years of age.
- No difference was observed between being vaccinated with two doses and having had a previous infection confirmed by PCR or antibody testing; That is, vaccines provide as much protection as having had COVID.
Conclusion Vaccination with a single dose of the Oxford-AstraZeneca or Pfizer-BioNTech vaccines, or two doses of Pfizer-BioNTech, significantly reduced new SARS-CoV-2 infections in this large community surveillance study. Greater reductions in symptomatic infections and/or infections with higher viral load are reflected in reduced rates of hospitalizations/deaths, but highlight the potential for limited continued transmission of asymptomatic infections in vaccinated individuals. |
Registration: The study is registered in the ISRCTN Registry, ISRCTN21086382.