Background
Testosterone treatment in men with hypogonadism improves bone density and quality, but trials with a sufficiently large sample size and long enough duration are needed to determine the effect of testosterone on fracture incidence.
Methods
In a subtrial of a double-blind, randomized, placebo-controlled trial evaluating the cardiovascular safety of testosterone treatment in middle-aged and older men with hypogonadism, we examined the risk of clinical fracture in a time-to-event analysis.
Eligible men were between 45 and 80 years of age with pre-existing cardiovascular disease or at high risk for it; one or more symptoms of hypogonadism; and two morning testosterone concentrations less than 300 ng per deciliter (10.4 nmol per liter), in fasting plasma samples obtained at least 48 hours apart.
Participants were randomly assigned to apply either a testosterone gel or a placebo gel daily.
At each visit, participants were asked if they had had a fracture since the previous visit. If they had them, medical records were obtained and adjudicated.
Results
The full analysis population included 5204 participants (2601 in the testosterone group and 2603 in the placebo group).
After a median follow-up of 3.19 years, a clinical fracture had occurred in 91 participants (3.50%) in the testosterone group and 64 participants (2.46%) in the placebo group (hazard ratio , 1.43; 95% confidence interval, 1.04 to 1.97).
The incidence of fractures also appeared to be higher in the testosterone group for all other fracture endpoints.
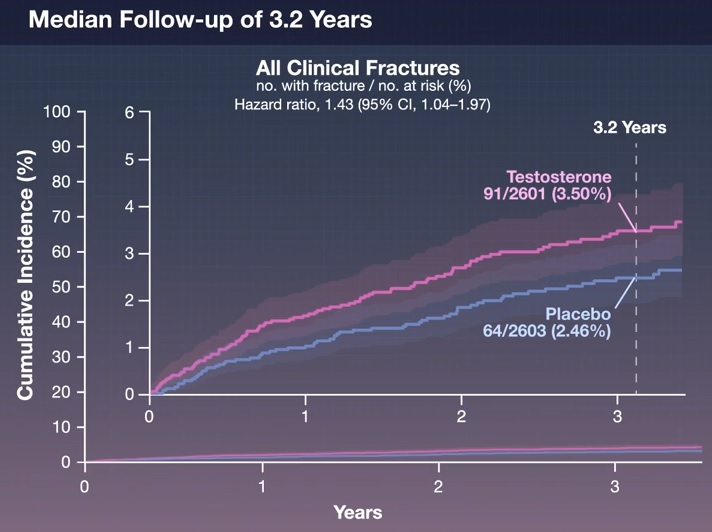
Conclusions Among middle-aged and older men with hypogonadism, testosterone treatment did not result in a lower incidence of clinical fracture than placebo. The incidence of fractures was numerically higher among men who received testosterone than among those who received placebo. |
(Funded by AbbVie et al.; TRAVERSE ClinicalTrials.gov number, NCT03518034)















