Almost all patients with vaccine-induced immune thrombotic thrombocytopenia had antibodies to platelet factor 4, as occurs with the rarely reported heparin-induced autoimmune thrombocytopenia.
Recent reports have described a syndrome involving thrombosis with thrombocytopenia and coagulopathy following administration of the AstraZeneca vaccine against SARS-CoV-2 (NEJM JW Infect Dis May 2021 and N Engl J Med 2021 Apr 9; [e-pub]) ; Similar events have been reported in several patients who received the Johnson & Johnson vaccine in the US.
To better understand the mechanism of this vaccine-induced immune thrombotic thrombocytopenia (VITT), researchers studied 23 patients (22 with thrombosis and thrombocytopenia; 1 with thrombocytopenia and a marked increase in D-dimer).
Key findings included:
|
Summary
Background
The pillar of control of the coronavirus disease 2019 (Covid-19) pandemic is vaccination against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). In one year, several vaccines have been developed and millions of doses administered. Reporting adverse events is a critical postmarketing activity.
Methods
We report findings in 23 patients who presented with thrombosis and thrombocytopenia 6 to 24 days after receiving the first dose of the ChAdOx1 nCoV-19 vaccine (AstraZeneca). On the basis of its clinical and laboratory features, we identified a novel underlying mechanism and addressed therapeutic implications.
Results
In the absence of prior prothrombotic medical conditions , 22 patients presented with acute thrombocytopenia and thrombosis, primarily cerebral venous thrombosis, and 1 patient presented with isolated thrombocytopenia and a hemorrhagic phenotype.
All patients had low or normal fibrinogen levels and elevated D-dimer levels at presentation.
No evidence of thrombophilia or causal precipitants was identified.
The platelet factor 4 (PF4) antibody test was positive in 22 patients (with 1 equivocal result) and negative in 1 patient.
On the basis of the pathophysiological characteristics observed in these patients, we recommend that treatment with platelet transfusions be avoided due to the risk of progression of thrombotic symptoms and that administration of a non-heparin anticoagulant agent and intravenous immunoglobulin be considered for the first onset. of these symptoms.
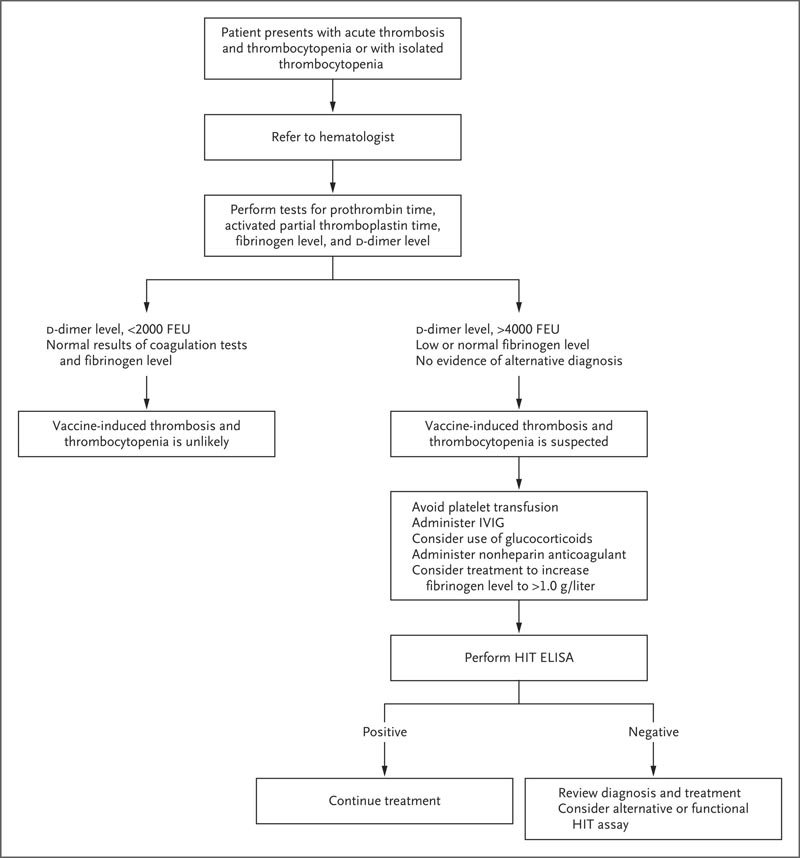
Suggested algorithm for testing and treatment of patients who develop thrombosis and thrombocytopenia 5 to 30 days after vaccination. The HemosIL AcuStar HIT IgG assay is not recommended for the evaluation of suspected vaccine-induced thrombosis and thrombocytopenia. ELISA denotes enzyme-linked immunosorbent assay, fibrinogen equivalent units FEU, heparin-induced thrombosis HIT, and intravenous immunoglobulin IVIG.
Conclusions Vaccination against SARS-CoV-2 remains essential for the control of the Covid-19 pandemic. A PF4-dependent pathogenic syndrome, unrelated to the use of heparin therapy, may occur after administration of the ChAdOx1 nCoV-19 vaccine. Rapid identification of this rare syndrome is important because of the therapeutic implications. |
Comment
The investigators provide a mechanistic explanation for VITT, which approximates rare reports of heparin-induced spontaneous autoimmune thrombocytopenia.
They present a management algorithm for suspected cases that involves avoidance of platelet transfusions, administration of intravenous immunoglobulin, consideration of steroids, use of a nonheparin anticoagulant, and consideration of correcting fibrinogen deficiency.
It is unclear whether patient outcomes will improve with these measures, but the authors note progressive thrombosis in some patients with early exposures to platelet and heparin transfusions. Unfortunately, although the majority of patients were younger women, there are no clear predisposing risk factors.