Background
Carbapenem-resistant Enterobacterales species and multidrug-resistant Pseudomonas aeruginosa are threats to global health. Cefepime-taniborbactam is an investigational combination of β-lactam and β-lactamase inhibitor with activity against Enterobacterales and P. aeruginosa species that express serine and metallo-β-lactamases.
Methods
In this randomized, double-blind, phase 3 trial, we assigned hospitalized adults with complicated urinary tract infection (UTI), including acute pyelonephritis, in a 2:1 ratio to receive intravenous cefepime-taniborbactam (2.5 g) or meropenem (1 g) every 8 hours for 7 days; This duration could be extended to 14 days in case of bacteremia.
The primary outcome was microbiological and clinical success (composite success) on days 19 to 23 of the trial in the microbiological intention-to-treat (microITT) population (patients who had a qualified gram-negative pathogen against which both study drugs were active). . A prespecified superiority analysis of the primary outcome was performed after confirmation of noninferiority.
Results
Of the 661 patients who underwent randomization, 436 (66.0%) were included in the microITT population. The mean age of the patients was 56.2 years and 38.1% were 65 years or older. In the microITT population, 57.8% of patients had complicated UTI, 42.2% had acute pyelonephritis, and 13.1% had bacteremia.
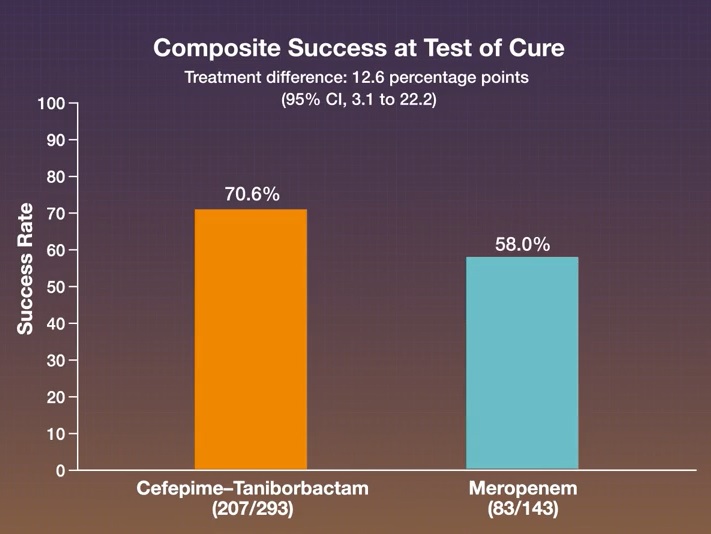
Combined success occurred in 207 of 293 patients (70.6%) in the cefepime-taniborbactam group and in 83 of 143 patients (58.0%) in the meropenem group. Cefepime-taniborbactam was superior to meropenem with respect to the primary outcome (treatment difference, 12.6 percentage points; 95% confidence interval, 3.1 to 22.2; P = 0.009).
Differences in treatment response were maintained at late follow-up (test days 28 to 35), when cefepime-taniborbactam had greater composite success and clinical success.
Adverse events occurred in 35.5% and 29.0% of patients in the cefepime-taniborbactam group and the meropenem group, respectively, with headache, diarrhea, constipation, hypertension, and nausea being the most frequently reported; The frequency of serious adverse events was similar in the two groups.
Conclusions Cefepime-taniborbactam was superior to meropenem for the treatment of complicated UTIs including acute pyelonephritis, with a safety profile similar to that of meropenem. |
(Funded by Venatorx Pharmaceuticals and others; CERTAIN-1 ClinicalTrials.gov number, NCT03840148. opens in new tab.)















