A drug that binds to allergy-causing antibodies may protect children from dangerous reactions when accidentally eating allergy-triggering foods, a new study found.
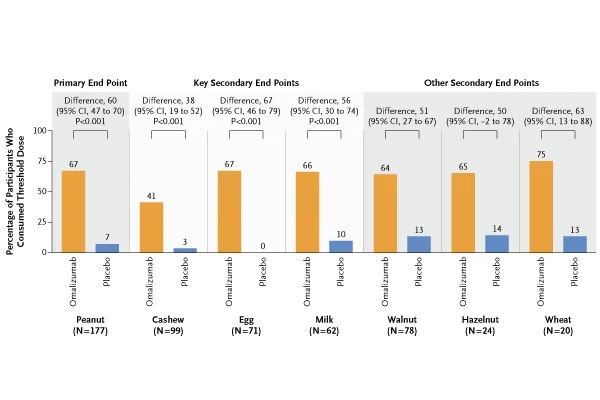
Summary Background Food allergies are common and associated with substantial morbidity; The only approved treatment is oral immunotherapy for peanut allergy. Methods In this trial, we evaluated whether omalizumab, an anti-IgE monoclonal antibody, would be effective and safe as monotherapy in patients with multiple food allergies. People aged 1 to 55 years who were allergic to peanuts and at least two other foods specified in the trial (cashews, milk, egg, walnuts, wheat, and hazelnuts) were examined. Inclusion required a reaction to a food challenge of 100 mg or less of peanut protein and 300 mg or less of the other two foods. Participants were randomly assigned, in a 2:1 ratio, to receive omalizumab or placebo administered subcutaneously (with dosing based on weight and IgE levels) every 2 to 4 weeks for 16 to 20 weeks, then of which the challenges were repeated. The primary endpoint was ingestion of peanut protein in a single dose of 600 mg or more without dose-limiting symptoms. The three key secondary endpoints were consumption of cashew, milk, and egg in single doses of at least 1000 mg each without dose-limiting symptoms. The first 60 participants (59 of whom were children or adolescents) who completed this first stage were enrolled in a 24-week open-label extension. Results Of the 462 people who were examined, 180 were randomized. The analysis population was made up of 177 children and adolescents (from 1 to 17 years of age). A total of 79 of 118 participants (67%) who received omalizumab met the primary endpoints, compared with 4 of 59 participants (7%) who received placebo (P<0.001). The results of the key secondary endpoints were consistent with those of the primary endpoint (cashews, 41% vs. 3%; milk, 66% vs. 10%; egg, 68% vs. 0%; P<0.001 for all comparisons). Safety endpoints did not differ between groups, other than more injection site reactions in the omalizumab group.
Conclusions In people as young as 1 year of age with multiple food allergies, treatment with omalizumab for 16 weeks was superior to placebo in increasing the reaction threshold to peanuts and other common food allergens. (Funded by the National Institute of Allergy and Infectious Diseases and others; ClinicalTrials.gov number, NCT03881696. opens in new tab.) |
Comments
Drug limits dangerous reactions to allergy-causing foods, according to study in children led by Stanford Medicine
A drug may make life safer for children with food allergies by preventing dangerous allergic responses to small amounts of allergy-triggering foods, according to a new study led by scientists at Stanford School of Medicine.
The research was published in the New England Journal of Medicine . The findings suggest that regular use of the drug omalizumab could protect people from serious allergic responses, such as difficulty breathing, if they accidentally eat a small amount of a food they are allergic to.
"I’m excited that we have a promising new treatment for patients allergic to multiple foods. This new approach showed really excellent responses for many of the foods that trigger allergies," said the study’s senior author, Sharon Chinthrajah, MD, associate professor of medicine. . and pediatrics, and interim director of the Sean N. Parker Center for Allergy and Asthma Research at Stanford Medicine.
"Patients affected by food allergies face a daily threat of life-threatening reactions due to accidental exposures," said the study’s senior author, Robert Wood, MD, professor of pediatrics at the Johns Hopkins University School of Medicine. "The study showed that omalizumab can be a layer of protection against small, accidental exposures."
Omalizumab, which the Food and Drug Administration originally approved to treat diseases such as allergic asthma and chronic hives , binds to and inactivates antibodies that cause many types of allergic diseases. Based on data collected in the new study, the FDA approved omalizumab on February 16 to reduce the risk of allergic reactions to food.
All study participants were severely allergic to peanuts and at least two other foods. After four months of monthly or bimonthly injections of omalizumab, two-thirds of the 118 participants who received the drug safely ate small amounts of the foods they were allergic to. Notably, 38.4% of study participants were under 6 years old , an age group at high risk of accidentally ingesting foods that trigger allergies.
Allergies are common
Food allergies affect approximately 8% of children and 10% of adults in the United States. People with severe allergies are recommended to completely avoid foods that contain their allergy triggers, but common allergens like peanuts, milk, eggs, and wheat can be hidden in so many places that everyday activities, such as attending going to parties and eating in restaurants can be a challenge.
"Food allergies have significant social and psychological impacts, including the threat of allergic reactions following accidental exposures, some of which can be life-threatening," Chinthrajah said. Families also face economic impacts from purchasing more expensive foods to avoid allergens, she added.
In the best available treatment for food allergies, oral immunotherapy , patients eat small, gradually increasing doses of allergy-triggering foods under a doctor’s supervision to develop tolerance. But oral immunotherapy itself can trigger allergic responses, desensitization to allergens can take months or years, and the process is especially long for people with multiple food allergies, since they usually receive treatment for one allergy at a time. Once they have been desensitized to an allergen, patients must also continue to eat the food regularly to maintain their tolerance to it, but people often do not like foods that they were long forced to avoid.
"There is a real need for treatment that goes beyond surveillance and offers options to our food-allergic patients," Chinthrajah said.
Omalizumab is an injected antibody that binds to and deactivates all types of immunoglobin E, or IgE, the allergy-causing molecule in the blood and immune cells of the body. So far, omalizumab appears capable of relieving multiple food allergens at once.
"We think it should have the same impact regardless of what food it is," Chinthrajah said.
Injections prevent serious reactions
The study included 177 children with at least three food allergies each, of whom 38% were between 1 and 5 years old, 37% between 6 and 11 years old, and 24% were 12 years old or older. Participants’ severe food allergies were verified by skin testing and food challenges; They reacted to less than 100 milligrams of peanut protein and less than 300 milligrams of each of the other foods.
Two-thirds of participants were randomly assigned to receive omalizumab injections and one-third received a placebo injection; The injections were performed for 16 weeks. Medication doses were set based on each participant’s body weight and IgE levels, and injections were administered once every two to four weeks, depending on the dose needed. Participants were retested between weeks 16 and 20 to see how much of each allergy-triggering food they could safely tolerate.
Upon retesting, 79 patients (66.9%) who had taken omalizumab could tolerate at least 600 mg of peanut protein, the amount in two or three peanuts, compared with only four patients (6.8%). %) who received the placebo. Similar proportions of patients showed improvements in their reactions to the other foods in the study.
About 80% of patients taking omalizumab were able to consume small amounts of at least one allergy-triggering food without inducing an allergenic reaction, 69% of patients were able to consume small amounts of two allergenic foods, and 47% of patients were able to eat small amounts of the three allergenic foods.
Omalizumab was safe and caused no side effects, apart from a few cases of minor injection site reactions. This study marks the first time its safety has been evaluated in children as young as 1 year old.
More questions
More research is needed to better understand how omalizumab could help people with food allergies, the researchers said.
"We have many unanswered questions: How long should patients take this medication? Have we permanently changed the immune system? What factors predict which people will have the strongest response?" Chintrajah said. "We do not know yet".
The team is planning studies to answer these and other questions, such as finding what type of follow-up would be necessary to determine when a patient acquires a significant tolerance to an allergy-triggering food.
Many patients who have food allergies also experience other allergic conditions treated with omalizumab, Chinthrajah noted, such as asthma, allergic rhinitis (hay fever and allergies to environmental triggers such as mold, dogs or cats, or dust mites), or eczema. "A drug that could improve all of their allergic conditions is exactly what we’re hoping for," she said.
The drug could be especially useful for young children with severe food allergies, he added, because they tend to put things in their mouths and may not understand the dangers posed by their allergies, he added.
The drug could also make it safer for community doctors to treat patients with food allergies, since it cannot trigger dangerous allergic reactions, as oral immunotherapy sometimes does. "This is something our food allergy community has been waiting for for a long time," Chinthrajah said. "It is an easy drug regimen to implement in medical practice and many allergists are already using it for other allergic conditions."
The research team included scientists from Johns Hopkins University School of Medicine, National Institutes of Allergy and Infectious Diseases, Icahn School of Medicine at Mount Sinai, Massachusetts General Hospital, University of Carolina School of Medicine of the North, University of Arkansas for Medical Sciences and Arkansas Children’s Hospital, Emory University School of Medicine and Children’s Healthcare of Atlanta, University of Texas Southwestern Medical Center, Perelman School of Medicine at the University of Pennsylvania, Genentech/Roche , Novartis Pharmaceuticals Corporation and Rho, Inc.
The research was funded by the National Institute of Allergy and Infectious Diseases and the National Center for Advancing Translational Sciences, both part of the National Institutes of Health (grant numbers UM2AI130836, UM1AI130838, UL1TR003098, UM1TR004408, UM1AI130570, UM1AI130839, UM1AI13093 6 , UM1 TR004406, UL1TR002535, UM1TR004399, UL1TR001878, UM1AI130781, UL1TR002378 and UL1TR003107), and the Claudia and Steve Stange Family Fund Genentech/Novartis provided research output and monetary support to Johns Hopkins University and collaborated in the study design .