The case of a 14-year-old boy with Down Syndrome is presented, who has suffered from a large purulent oral discharge for six years, clinically evident upon waking up.
Several studies demonstrated the presence of normal microbiota and with Candida ssp . During all this time, the condition was refractory to conventional antimicrobial and antifungal treatments.
The mucosa of the lips and tongue shows papillomatous lesions compatible with those observed in Cowden Syndrome.
The discharge material was studied and through typing Candida dubliniensis was diagnosed , also determining the sensitivity to voriconazole with the antifungal susceptibility method.
The discharge resolved with treatment based on voriconazole.
The aim is to highlight the presence of purulent material of fungal origin and the importance of antifungal typing and susceptibility in cases resistant to fluconazole.
| Case report |
The case corresponds to a 14-year-old boy, with Down Syndrome, who is referred to the clinic, with an evolution of six years, after receiving different therapies without success.
The child suffered from a purulent discharge, very noticeable in the mornings when he woke up, coming out of the mouth through the corner, spilling onto the skin of the face and the pillow (Fig. 1).
The different microbiological and mycological studies that were carried out during the six years of the disease reported: normal microbiota and the presence of Candida ssp . With that diagnosis, he received large doses of antibiotics and antifungals for long periods to no avail. He was also prescribed mouthwashes of different characteristics with no response.
He is celiac and diabetic, with no other alterations to highlight, beyond the characteristics of his underlying disease.
He reported oral surgery in a hospital to remove a dentigerous cyst on the palate in zone 22, at the age of 8, without postoperative complications.
The characteristic of the labial and lingual mucosa is striking, which presents an irregular surface with a papillomatous appearance, leaving deep grooves between the elevations, especially on the lower lip (Fig. 2).
Although, as is known, the anatomical structure of the mouth in this syndrome presents with differentiated characteristics, the mother assures that this aspect of the mucosa coincides with the appearance of the nocturnal purulent discharge. She also associates the origin of the condition with the time of her hospitalization for dentigerous cyst surgery. There is a great clinical coincidence with the mucosal lesions observed in Cowden syndrome.
Lip and tongue biopsies are indicated, with HE and PAS stains, whose pathological report indicates: Inflammatory Epithelial Hyperplasia associated with Chronic Candidiasis.
The thyroid, digestive tract and skin were studied at the same time to rule out lesions associated with Cowden Syndrome, and no evidence of disease was found.
To genetically investigate whether the patient suffered from Cowden Syndrome, a molecular study was requested by sequencing and microarray of the PTEN gene, extracting DNA from peripheral blood, PCR amplification with specific primers for each axon and purification. Analysis of the sequences of the amplified products of each exon was carried out with ABI PRIM 310 genetic analyzer. The result reports that in exons 5,6,7,8, WILD TYPE, no alterations were observed.
Trying to identify the original focus that produced the discharge, clinical analysis, CT, MRI of the solid skull, face, neck and thorax, panoramic the case.
An Immunological Profile is indicated which reports that although some immunological alterations are observed, all of them are not significant. APECED (autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy) is ruled out. Parathyroid hormone levels are normal. The absence of a family history of candidiasis excludes defects in STAT 1.
Although the previous and current microbiological studies provided by the child’s mother were carried out in reliable laboratories, it was decided to examine the child when he woke up and collect material from the discharge again.
The sample obtained consisted of a purulent secretion and its processing was carried out in the Diagnostic Laboratory of the Chair of Microbiology and Parasitology of the Faculty of Dentistry of the University of Buenos Aires.
Direct examinations were performed using the sample using Gram and Giemsa staining. Microscopic observation revealed few gram-negative bacilli, gram-positive diplococci and a fair amount of yeast cells with pseudomycelium. A positive inflammatory response was observed with more than 5 polymorphonuclear neutrophils per field. 100 μl of the sample was seeded by dispersion with a Drigalski spatula in the following culture media: Levine; CLDE; IHB; CHROMagar Candida® (CA) and incubated under aerobic conditions at 37ºC for 48 hours. Additionally, the sample was sown on Lacquered Blood Agar medium under anaerobic conditions at 37ºC for 7 days. Colonies of yeast cells were recovered exclusively from all cultured media.
In the CA medium, 105 CFU/ml of green colonies of Candida spp were isolated . Phenotypic and micromorphological methods were used for typing. Microcultures were carried out on Milk Agar of the isolated colony and the formation of germ tube was observed after 3 hours of incubation at 37°C and the presence of pseudohyphae and chlamyconidia after 48 hours at 28°C. For the differentiation between the species of Candida albicans and Candida dubliniensis , the strain was plated on Staib Agar (AS) at 30ºC for 4 days and on Sabouraud glucose agar (ASG) at 45ºC for 48 hours. In the AS medium, the presence of pseudohyphae and chlamyconidia was observed and no development was obtained in the ASG.
The disk diffusion method according to CLSI document M44-A2 was used to perform antifungal susceptibility to the drugs fluconazole and voriconazole.
According to the tests carried out, the strain studied was phenotyped as
Candida dubliniensis , which was susceptible to voriconazole and resistant to fluconazole.
| Discussion |
At the same time that the origin of the discharge was searched, Florida Oral Papillomatosis was ruled out. Eversole describes a case of POF associated with Down Syndrome 1. The pathological anatomy was negative.
Cowden syndrome is an autosomal dominant disorder characterized by multiple hamartomatous tumors of ectodermal, mesodermal, and endodermal origin. The disorder is due to mutations of the PTEN gene. The genetic study was also negative. Torre and Cruces publish a case of Cowden Syndrome and Down Syndrome 2 .
The continuity and resistance to therapy of the purulent discharge was surprising. In the case of a child with Down Syndrome, the first line of defense against bacteria and viruses, that is, innate immunity, is clearly qualitatively and quantitatively altered. The number of NK cells is often decreased and phagocytosis and chemotaxis of polymorphonuclear cells and monocytes are reduced, thereby decreasing the ability to directly kill pathogens.
The second line of defense, T cell-mediated adaptive immunity, is also usually altered in Down syndrome. There is reduced production in DC4 and CD8 T cells, and a lower level of cytotoxicity required to deal with pathogens. There is a reduction in B lymphocytes, and although immunoglobulin levels may not be decreased, antibody-dependent cytotoxicity mediated by NK cells is depressed 3,4,5 .
Children with Down Syndrome also present a chromosomal alteration that affects the anatomical structure of the mouth, leading to a greater capacity for Candida to adhere to the affected mucosa. These facts make children with this syndrome great "loaders" of yeast. predisposing them to oral candidiasis and other infections 3 .
Although Candida albicans has been the most frequent agent in oropharyngeal candidiasis in immunosuppressed patients, in recent years its prevalence has decreased, favoring other species such as C. tropicalis, C. glabrata, C. krusei and, since 1996, the new species called C. .dubliniensis .
In general, these yeasts are considered normal microbiota of the human oral cavity. Therefore, competition will be established between C. albicans and C. dubliniensis 8
This prolonged use of antifungals can eliminate the competition of C. dubliniensis with other fungi (mainly C. albicans ) and increase the risk of oropharyngeal candidiasis 9 . Due to its great similarity to C. albicans , it is possible that there is under-reporting of prevalent cases.
In some studies, C. dubliniensis candidiasis has been associated with other factors such as history of intravenous drug use, underlying debilitating diseases that were previously treated (for a period of no more than 9 months) with fluconazole , HIV-negative patients, but with underlying debilitating diseases such as decompensated diabetes and use of immunosuppressive drugs 10 .
In-hospital infection by Candida dubliniensis has been described , which does not allow us to rule out that in this case it may have started when the patient underwent surgery for his palate lesion, as his mother maintained 12 .
In the case presented, the papillomatous folds caused by the action of the chronic fungal infection constituted the ideal reservoir to keep the infection active for so many years. Fig 3
Voriconazole, the drug of choice in this case, along with posaconazole and ravuconazole, are fluorinated triazole derivatives called “second generation azoles”, like fluconazole and itraconazole.
Like all azoles, triazole derivatives exert their activity through the inhibition of some of the numerous steps leading to the synthesis of ergosterol and other sterols. These compounds are fundamental structural components of the fungal membrane and each of them has specific functions. It is important to monitor possible side effects of the drug.
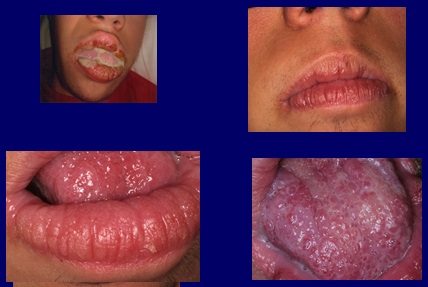
Treatment was started with voriconazole 200 mg tablets, two per day for four months, obtaining a reversal of the symptoms, the discharge disappearing and the clinical appearance of the mucosa improving (Fig. 4). A serological clinical control was carried out to control possible side effects of the drug, which fortunately did not occur.
| Conclusion |
Candida dubliniensis has emerged as an important pathogen, especially in immunocompromised patients with manifestations mainly in the oral cavity.
The patient with Down Syndrome may present immunological disorders and special anatomical conditions in the oral cavity that make them great “ carriers” of yeast.
Candida dubliniensis presents certain difficulties in its identification, particularly when it comes to being differentiated from Candida albicans . This differentiation is important due to differences in resistance to antifungal treatment. Even so, there are simple biochemical and phenotypic tests that allow these yeasts to be separated.
It is noteworthy that the presence of purulent material in the discharge leads us, in the first instance, to indicate antibiotic therapy, as happened in the case presented, but in the absence of response to said therapy, we must think about the fungal nature of the infection and select the indicated antifungal 13,14, 15.16
Fig 1 Large purulent discharge, clinically visible upon awakening
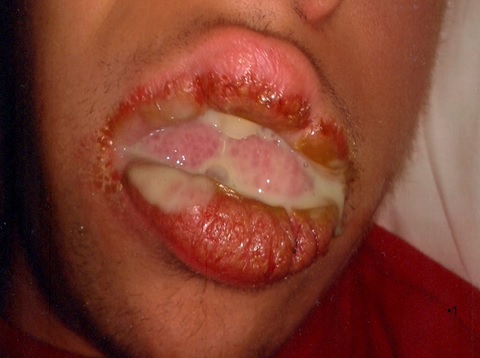
Fig 2 The mucosa of the lip and tongue shows papillomatous images compatible with Cowden syndrome.
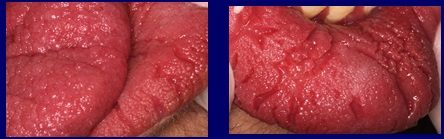
Fig3 The papillomatous folds and grooves caused by the action of the chronic fungal infection constitute the ideal reservoir to keep the infection active.
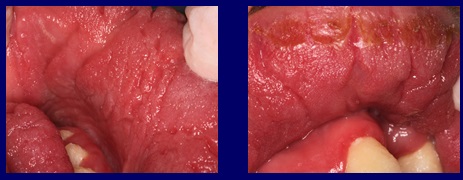
Fig4 After treatment with voriconazole, 200 milligrams twice a day for four months, the oral purulent discharge disappears and the texture of the mucosa improves.
Authors: Ceccotti EL1, Turcot L2, Molgatini S2Gliosca L2, Fedelli L2, Bruzzone R.3
1 National Academy of Dentistry
2 Chair of Microbiology and Parasitology of the Faculty of Dentistry of the University of Buenos Aires.
3 Former Prof, Head of Stomatology Clinic. USAL.AOA















