On December 20, 2020, Israel began a national vaccination program against COVID-19. One prioritized group was health care workers, many of whom are breastfeeding women.
Although the vaccine trial did not include this population and no other safety data related to the vaccine had been published, breastfeeding women in risk groups were encouraged to receive the vaccine. The Centers for Disease Control and Prevention has also recommended that breastfeeding women in the vaccine’s target groups be immunized.
We investigated whether maternal immunization results in the secretion of SARS-CoV-2 antibodies into breast milk and evaluated any potential adverse events between women and their babies.
Methods
We conducted a prospective cohort study of a convenience sample of breastfeeding women (either exclusively or partially) from vaccine-targeted groups who chose to be vaccinated. Participants were recruited from all over Israel between December 23, 2020 and January 15, 2021, through advertisements and social media.
All participants received 2 doses of the Pfizer-BioNTech vaccine 21 days apart. Breast milk samples were collected before vaccine administration and then once a week for 6 weeks beginning at week 2 after the first dose.
Samples were kept frozen pending analysis. IgG levels were detected using the Elecsys Anti - SARS-CoV-2 S serological assay and read on the Cobas e801 analyzer with a level of more than 0.8 U/ml considered positive (La Roche Ltd) and IgA with the EUROIMMUN AG Anti-SARS -Kit CoV-2 S with an extinction ratio of samples over calibrator greater than 0.8 considered positive (Supplement).
At enrollment, maternal and infant demographic information was collected, followed by weekly questionnaires in conjunction with breast milk collection requesting information on interim well-being and vaccine-related adverse events.
The study was approved by the Shamir Medical Center Institutional Review Board; Written informed consent was obtained from the mothers.
Changes in the proportion of participants testing positive and in antibody levels during the study were assessed using paired-sample t tests, comparing antibody levels at each time point with baseline and correcting for multiple testing using the Benjamini-Hochberg procedure. A two-sided significance threshold was set at P < 0.05.
Results
Eighty-four women completed the study, providing 504 breast milk samples. Women had a mean (SD) age of 34 (4) years and infants 10.32 (7.3) months.
Mean levels of specific anti-SARS-CoV-2 IgA antibodies in breast milk increased rapidly and were significantly elevated 2 weeks after the first vaccination (ratio 2.05; p < 0.001), when 61.8% of samples tested positive, increasing to 86.1% at week 4 (1 week after second vaccine).
Mean levels remained elevated throughout the duration of follow-up, and at week six, 65.7% of samples tested positive. Specific anti-SARS-CoV-2 IgG antibodies remained low during the first 3 weeks, with an increase at week 4 (20.5 U/mL; P = 0.004), when 91.7% of samples tested positive , increasing to 97% in weeks 5 and 6 (Figure).
No mother or baby experienced any serious adverse events during the study period. Forty-seven women (55.9%) reported a vaccine-related adverse event after the first vaccine dose and 52 (61.9%) after the second dose, with local pain being the most common complaint.
Four infants developed fever during the study period 7, 12, 15, and 20 days after maternal vaccination. All had symptoms of upper respiratory tract infection, including cough and congestion, which resolved without treatment, except for one infant who was admitted for evaluation of neonatal fever due to her age and was treated with antibiotics pending culture results. . No other adverse events were reported.
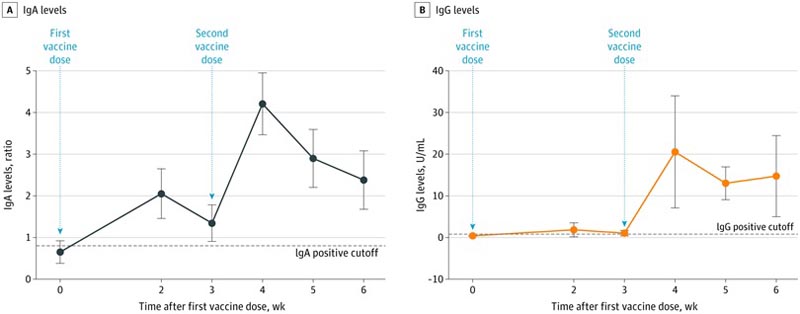
A, All comparisons between time points are P < 0.001. B, The comparison point at week 4 is P = 0.004; at week 5, p < 0.001; and at week 6, p = 0.005.
Discussion
This study found strong secretion of SARS-CoV-2-specific IgA and IgG antibodies in breast milk for 6 weeks after vaccination.
IgA secretion was evident as early as 2 weeks after vaccination followed by a peak in IgG after 4 weeks (one week after the second vaccination). Some other studies have shown similar findings in women infected with COVID-19.5.
The antibodies found in the breast milk of these women showed strong neutralizing effects, suggesting a possible protective effect against infection in the infant.
The study has limitations. First, no functional assays were performed. However, previous studies have shown neutralizing capabilities of the same antibodies measured for this study. Second, no serum antibody testing or real-time reverse transcriptase polymerase chain reaction testing of SARS-CoV-2 was performed, which would have provided interesting correlations.