Protection against symptomatic COVID-19 was >60% 28 days after a dose of either vaccine, and if infection developed despite vaccination, the clinical course was attenuated.
The Phase 3 trials supporting the authorization of the currently available COVID-19 vaccines are well known, but how effective are these vaccines in real life, especially when a single dose is administered (as has been recommended in the UK ) to allow for broader coverage with limited supplies?
Researchers conducted a population-based study on the effectiveness of COVID-19 vaccination in England between December 8, 2020 and February 19, 2021, when the B1.1.7 variant emerged. SARS-CoV-2 PCR test results in 156,930 adults aged 70 years and older were linked to national vaccination and mortality registries and hospital admission data.
When vaccination status was evaluated in people who tested positive for SARS-CoV-2 (44,590; 28%) compared to those who tested negative (112,340; 72%), a protective effect was observed in Pfizer/ BioNTech BNT162b2 10 to 13 days after vaccination, reaching 61% effectiveness at 28 days .
For those who received the AstraZeneca vaccine (ChAdOx1-S), protective effects emerged 14 to 20 days after vaccination, reaching 60% effectiveness after 28 days.
Among individuals aged 80 years and older who received BNT162b2, vaccine efficacy was 70% 28 days after the first dose and 89% 14 days after the second dose.
If infection developed despite vaccination ≥14 days earlier, the incidence of emergency hospital admission was 43% lower with BNT162b2 and 37% lower with ChAdOx1-S. For BNT162b2, the risk of mortality within 21 days was reduced by 53%.
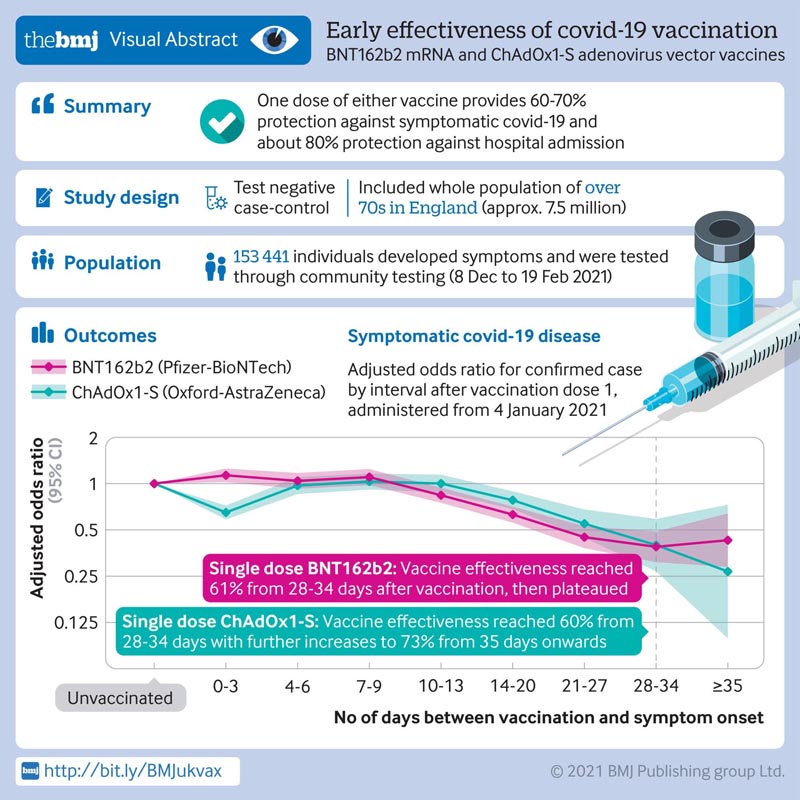
Conclusion Vaccination with one dose of BNT162b2 or ChAdOx1-S was associated with a significant reduction in symptomatic COVID-19 in older adults and greater protection against severe disease. Both vaccines showed similar effects. Protection was maintained during follow-up (>6 weeks). A second dose of BNT162b2 was associated with greater protection against symptomatic disease. A clear effect of vaccines against the B.1.1.7 variant was found. |
Comment
This large observational study reflects previous investigations of COVID-19 vaccine effectiveness in England and Scotland. Considerable protective effects are observed with the BNT162b2 and ChAdOx1-S vaccines, even after a single dose . However, a prime/boost strategy offers greater protection and is clearly preferable as long as vaccine supplies are sufficient.