Background
Prognoses regarding mortality and hepatic and nonhepatic outcomes across the histologic spectrum of nonalcoholic fatty liver disease (NAFLD) are not well defined.
Methods
We prospectively followed a multicenter patient population that included the entire histologic spectrum of NAFLD. Incidences of death and other outcomes were compared across baseline histologic characteristics.
Results
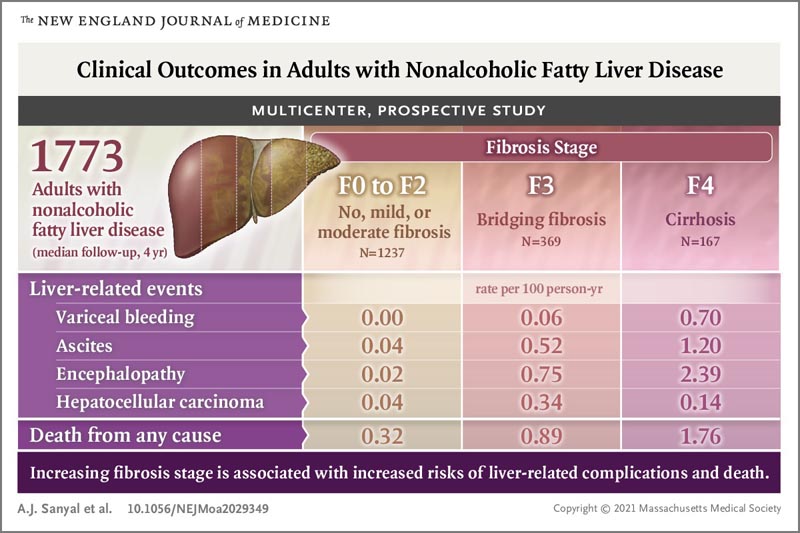
A total of 1773 adults with nonalcoholic fatty liver were followed for a median of 4 years.
All-cause mortality increased with increasing stages of fibrosis (0.32 deaths per 100 person-years for stages F0 to F2 [no fibrosis, mild or moderate], 0.89 deaths per 100 person- years for stage F3 [bridging fibrosis] and 1.76 deaths per 100 person-years for stage F4 [cirrhosis]).
The incidence of liver-related complications per 100 person-years increased with fibrosis stage (F0 to F2 vs. F3 vs. F4) as follows: variceal hemorrhage (0.00 vs. 0.06 vs. 0 .70), ascites (0.04 vs. 0.52 vs. F4). 1.20), encephalopathy (0.02 vs 0.75 vs 2.39) and hepatocellular cancer (0.04 vs 0.34 vs 0.14).
Compared with patients with stage F0 to F2 fibrosis, patients with stage F4 fibrosis also had a higher incidence of type 2 diabetes (7.53 vs. 4.45 events per 100 person-years) and a decrease in more of 40% in the estimated glomerular filtration rate (2.98 versus 0.97 events per 100 person-years).
The incidence of cardiac events and non-hepatic cancers was similar in all stages of fibrosis.
After adjustment for age, sex, race, diabetes status, and baseline histologic severity, the incidence of any hepatic decompensation event (variceal hemorrhage, ascites, or encephalopathy) was associated with increased all-cause mortality (IRR). adjusted risk, 6.8; 95%). confidence interval, 2.2 to 21.3).
Conclusions In this prospective study involving patients with NAFLD, fibrosis stages F3 and F4 were associated with an increased risk of liver-related complications and death. |
(Funded by the National Institute of Diabetes and Digestive and Kidney Diseases and others; NAFLD DB2 ClinicalTrials.gov number, NCT01030484)















