The fecal immunochemical test, or FIT, is a simple, non-invasive test for patients who present to their doctors with symptoms related to bowel cancer. It is a stool test that can reliably detect minute amounts of human blood in stool that is not always visible to the naked eye. Most bowel cancers bleed to varying degrees into the colon and mix with stool. FIT has been widely used in bowel cancer screening programmes, but surprisingly it is only with COVID restricting access to healthcare that interest has taken hold in expanding its clinical use for patients with symptoms of bowel cancer. We hypothesized that the FIT test could measure the amount of blood in the stool of patients presenting with concerning symptoms and classify them into different colorectal cancer risk groups to help physicians determine the need and urgency to recommend further investigation. invasive of the colon. |
Incidence and mortality from colorectal cancer are increasing in some countries. Population bowel screening programs have increased demand on limited endoscopy resources.
Patients with colorectal symptoms that raise the possibility of a colorectal cancer diagnosis also compete for endoscopy capacity, which can lead to delays in diagnosis and poorer treatment outcomes.
Symptoms alone are poor predictors of a colorectal cancer diagnosis. A previous systematic review concluded that a fecal immunochemical test (FIT) was clinically useful and cost-effective for triaging patients with symptoms of possible colorectal cancer.
This led to a change in the UK’s National Institute for Health and Care Excellence (NICE) recommendations to include the use of FIT in symptomatic patients. The COVID-19 pandemic has even more limited access to endoscopy, promoting increased interest in FIT as a triage tool for patients with colorectal symptoms.
Three previous meta-analyses of small cohort studies concluded that, at the fecal hemoglobin (f-Hb) threshold of 10 µg Hb/g stool, FIT is useful in excluding colorectal cancer in symptomatic patients.
However, larger cohort studies have since been published, so an updated meta-analysis is required. Additionally, more questions have been raised about the applicability of FIT in patients with specific symptoms, and there are conflicting reports on how FIT has influenced service delivery.
This review evaluated the utility of the single quantitative fecal immunochemical test (FIT) as a triage tool for patients with symptoms of possible colorectal cancer, the effect of symptoms on the accuracy of FIT, and the impact of incorporating FIT on the provision of services.
The primary objective of this meta-analysis was to evaluate the utility of quantitative FIT as a triage tool at lower f-Hb thresholds to exclude symptomatic patients from invasive investigations, and at higher f-Hb thresholds to prioritize the urgency of the investigation.
Secondary objectives were to evaluate whether specific symptoms have an impact on FIT diagnostic performance and to evaluate the impact of FIT classification on service delivery.
Methods
Five databases were searched. Meta-analyses were performed on the sensitivities and specificities of extracted FIT for colorectal cancer detection at the reported f-Hb thresholds. Secondary outcomes included the sensitivity and specificity of FIT for advanced colorectal neoplasia and severe bowel disease. A subgroup analysis was performed by FIT brand and symptoms.
Results
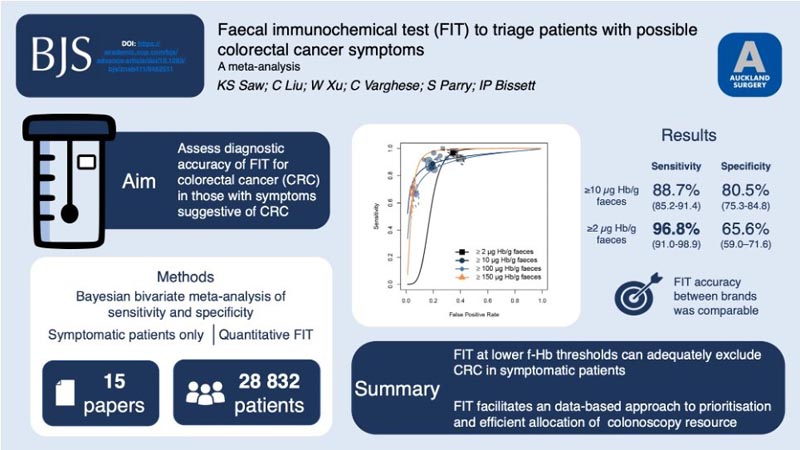
Fifteen prospective cohort studies were included, including 28,832 symptomatic patients. At the most frequently reported f-Hb positivity threshold of ≥ 10 µg Hb/g stool (n = 13), the summary sensitivity was 88.7% (95% ci 85.2 to 91.4) and specificity was 80.5% (95% ci 75.3 to 84.8) for colorectal cancer.
At the lower limits of detection of ≥ 2 µg Hb/g in stool, the summary sensitivity was 96.8% (95% ci 91.0 to 98.9) and the specificity was 65.6% (95% ci 59 .0 to 71.6).
At the upper f-Hb positivity thresholds of ≥ 100 µg Hb/g stool and ≥ 150 µg Hb/g stool, summary sensitivities were 68.1% (95% ci 59.2 to 75.9) and 66 .3% (95% ci 52.2 to 78.0), with specificities of 93.4% (95% ci 91.3 to 95.1) and 95.1% (95% ci 93.6 to 96, 3) respectively.
The sensitivity of FIT was comparable between different assay brands.
Discussion
Our analysis showed that at the lowest possible fecal blood detectable by FIT (≥2 micrograms Hb/g feces), a positive FIT test would detect approximately 96 out of 100 colorectal cancers. In two of the largest studies conducted in the United Kingdom, up to 63% of patients who, by current standards, would undergo invasive colonic investigation due to reported symptoms, could avoid it.
When settings are adjusted to be in line with current National Institute for Health and Care Excellence (NICE) recommendations (≥10 micrograms Hb/g stool), a positive FIT test would detect approximately 88 in 100 colorectal cancers.
If one has a negative FIT test in this setting, it is estimated that approximately only 1 in 243 patients undergoing invasive colonic investigation would have cancer detected, meaning that under contemporary practices, 242 patients would assume the risk of a colonoscopy. despite not having bowel cancer.
In contrast, if FIT detects higher levels of blood in stool (such as ≥100 or ≥150 micrograms Hb/g in stool), approximately 1 in 3 patients will be diagnosed with bowel cancer.
- For patients with symptoms, these results indicate that when FIT detects very low levels of blood in the stool, the chances of having bowel cancer are sufficiently low that invasive investigation can be avoided .
- On the contrary, if the FIT indicates relatively higher blood levels, the probability of bowel cancer is very high and urgent colon investigation would be ideal to detect and treat the cancer as early as possible.
No test is perfect. The currently accepted gold standard, colonoscopy, is estimated to miss 5 in 100 cancers. (4) With the correct configuration, our analysis suggests that FIT approaches this diagnostic accuracy without being invasive, accessible, and cheap. While colon imaging tests have an irreplaceable role in the diagnosis of bowel cancer, it may be more optimal for patients, physicians, and healthcare systems to rationalize the use of colonoscopy and colonography to avoid delays. in the diagnosis and treatment of those considered to be at increased risk of bowel cancer.
The results of this systematic review and meta-analysis suggest that a single quantitative FIT with low thresholds of f-Hb positivity can adequately exclude colorectal cancer in patients referred with symptoms of possible colorectal cancer.
More data on the performance of FIT at upper f-Hb thresholds may further validate its utility as a prioritization tool. FIT facilitates a data-driven approach to prioritization and more efficient allocation of colonoscopy resources.
These findings have the potential to reduce the rate of unnecessary colonic investigations and free up colonoscopy capacity for colorectal cancer screening and surveillance initiatives.
Conclusion Single quantitative FIT with lower f-Hb positivity thresholds can adequately exclude colorectal cancer in symptomatic patients and provides a data-driven approach to prioritizing colonoscopy resources. |
















