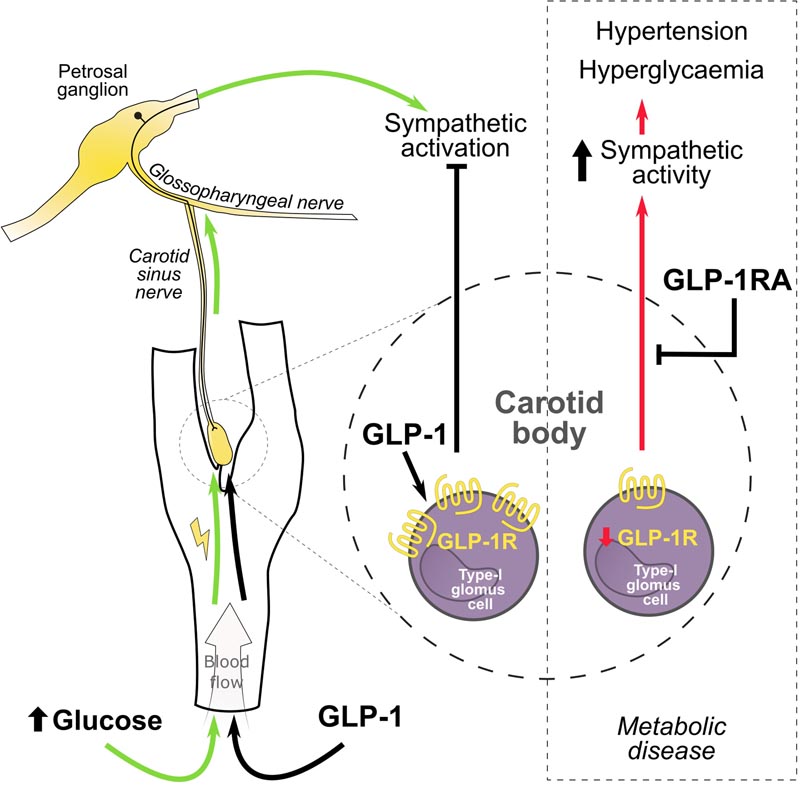
Summary GLP1R attenuates the sympathetic response to high blood glucose through inhibition of the carotid body Aberrant activity of the sympathetic nervous system exacerbates cardiovascular risk in hypertension and diabetes, which are common comorbidities, but clinically sympathetic activity remains poorly controlled. The diabetic and hypertensive state is associated with an increase in reflex sensitivity and tonic impulse of peripheral chemoreceptors, the cause of which is unknown. Previously, we have shown that hypertension critically depends on carotid body (CB) input in spontaneously hypertensive rats, a model that also exhibits a number of diabetic traits. Overstimulation of CB by insulin and leptin has similarly been implicated in the development of increased sympathetic nervous activity in metabolic syndrome and obesity. Therefore, we hypothesize that in the diabetic and hypertensive state (spontaneously hypertensive rat), the CB is sensitized by altered metabolic signaling that causes excessive levels of sympathetic activity and dysfunctional reflex regulation. Methods: Using an RNA-seq approach we investigated possible molecular targets involved in energy metabolism mediating CB sensitization and its regulation of sympathetic outflow in experimental hypertension. Identified targets were characterized using molecular and functional techniques that assess peripheral chemoreflex sensitivity in situ and in vivo . Results: We found the expression of GLP1R (glucagon-like peptide 1 receptor) in the CBs of rats and humans and that its decreased expression is related to sympathetic hyperactivity in rats with cardiometabolic disease. We demonstrated that GLP1R localizes to CB chemosensory cells, while targeted administration of GLP1R agonist to the CB reduced its basal discharge and attenuated chemoreflex-evoked blood pressure and sympathetic responses. Importantly, hyperglycemia-induced peripheral chemoreflex sensitization and associated basal sympathetic hyperactivity were abolished by GLP1R activation in the CB, suggesting a role in a homeostatic response to high blood glucose. Conclusions: We show that GLP1 (glucagon-like peptide-1) modulates the peripheral chemoreflex acting on the CB, supporting this organ as a multimodal receptor. Our findings point to CBs as potential targets to improve excessive sympathetic activity using GLP1R agonists in the hypertensive-diabetic condition. |
Comments
They discover a link between high blood pressure and diabetes
The long-standing enigma of why so many patients who suffer from high blood pressure also have diabetes has finally been solved by an international team led by the universities of Bristol, United Kingdom, and Auckland, New Zealand. .
Important new discovery has shown that a small cellular protein glucagon-like peptide-1 (GLP-1) combines the control of blood sugar and blood pressure.
Professor Julian Paton, lead author and director of Manaaki Mãnawa, the University of Auckland Heart Research Centre, said: "We have known for a long time that hypertension and diabetes are inextricably linked and we have finally discovered why we now will inform new treatment strategies.”
The research, published online ahead of print in Circulation Research, involved contributions from collaborating scientists in Brazil, Germany, Lithuania and Serbia, as well as the United Kingdom and New Zealand.
GLP-1 is released from the gut wall after eating and acts to stimulate insulin from the pancreas to control blood sugar levels. This was known, but what has now been discovered is that GLP-1 also stimulates a small sensory organ called the carotid body located in the neck.
The group at the University of Bristol used an unbiased, high-throughput genomics technique called RNA sequencing to read all the messages from genes expressed in the carotid body in rats with and without high blood pressure. This led to the finding that the receptor that detects GLP-1 is located in the carotid body, but less so in hypertensive rats.
David Murphy, Professor of Experimental Medicine at Bristol Medical School: Translational Health Sciences (THS) and senior author, explained: “Locating the link required genetic profiling and multiple validation steps. “We never expected GLP-1 to appear on the radar, so this is very exciting and opens up many new opportunities.”
Professor Paton added: “The carotid body is the convergent point where GLP-1 acts to simultaneously control blood sugar and blood pressure; This is coordinated by the nervous system which is instructed by the carotid body.”
People with hypertension and/or diabetes are at high risk of life-threatening cardiovascular diseases. Even when receiving medication, a large number of patients will remain at high risk.
This is because most medications only treat the symptoms and not the causes of high blood pressure and high sugar.
Professor Rod Jackson, world-renowned epidemiologist at the University of Auckland, said: "We knew that blood pressure is notoriously difficult to control in patients with high blood sugar, so these findings are really important because by giving "GLP-1 might be able to reduce sugar and blood pressure together, and these two factors are the main contributors to cardiovascular risk."
Mr Audrys Pauža, a British Heart Foundation-funded PhD student in Professor David Murphy’s laboratory at Bristol Medical School and lead author of the study, added: "The prevalence of diabetes and hypertension is increasing in everyone, and there is an urgent need to address this.
“Drugs targeting the GLP-1 receptor are already approved for use in humans and are widely used to treat diabetes. In addition to helping to lower blood sugar, these medications also lower blood pressure, however, the mechanism of this effect was not well understood.
“This research revealed that these drugs may actually work on the carotid bodies to exert their antihypertensive effect. Building on this work, we are already planning translational studies in humans to put this discovery into practice, so that patients at highest risk can receive the best available treatment.”
But GLP-1 is just the beginning. The research has revealed many novel targets for ongoing functional studies that the team anticipates will lead to future translational projects in human hypertensive and diabetic patients.
The study was funded by the British Heart Foundation and the Health Research Council of New Zealand.